Background
Intrahepatic cholangiocarcinoma (ICC) is arising from the intrahepatic bile ducts and accounts for 8%-10% of all malignant hepatic tumors. Cholangiocarcinomas are more frequent in men than in women (60%-70%), and their mean age at presentation is in the seventh decade [1]. Multiple histological variants have been described in cholangiocarcinoma-like mucinous, signet ring cell, adenosquamous, lymphoepithelioma-like, clear cell, spindle cell, as well as carcinomas with osteoclast-like giant cells and with papillary features [2–5].
A thorough review of the literature revealed only three such cases that have been previously reported [6–8]. We recently encountered a new morphological variant of ICC showing thyroid-like follicular pattern, which is described in this report.
Case Presentation
A 42-year-old female patient presented with complaints of pain in the right flank region for 15-20 days. Clinical examination revealed hepatomegaly and was otherwise unremarkable. She had no significant history or family history.
An abdominal computed tomography (CT) scan showed 89 mm × 55 mm × 74 mm well-defined heterogeneous lesion in segment V right lobe of the liver with solid cystic areas. Mild capsular retraction was noted. Lesion appeared to be neoplastic. The possibility of hepatocellular carcinoma was suggested on radiology. No other abnormal lesion was identified. Serum transaminases, alkaline phosphatase, and bilirubin profile were within normal limits. Serum alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) level were normal, whereas carbohydrate antigen 19-9 (CA 19-9) was elevated (731.30 U/ml; normal range: 0-37 U/ml).
Ultrasonography-guided biopsy was done. Findings were suggestive of low-grade malignant tumor having glandular and follicular pattern which were lined by low cuboidal type epithelial cells without intervening desmoplastic stroma. Possibilities were (1) metastatic tumor and (2) hepatocellular carcinoma with pseudoglandular pattern (Figure 1).
Immunohistochemistry (IHC) was given on biopsy section to differentiate these two possibilities. On IHC, tumor cells were cytokeratin 7 (CK 7) and CK 19 positive, whereas negative for hepatocyte paraffin 1(HepPar1), AFP, CEA, CK 20, caudal-related homeobox gene 2 (CDX2), paired box gene 8 (PAX8), and thyroid transcription factor 1 (TTF-1). Hence, the final diagnosis was offered as metastatic adenocarcinoma with primary origin likely to be from pancreaticobiliary tract.
Positron emission tomography-CT (PET-CT) scan was done showing hypodense lesion in segment V of the liver measuring 6.5 cm × 6.6 cm with minimal uptake. Mild adjacent capsular retraction was noted suggestive of primary hepatic mass. Few subcentimeter nodes were identified in mid and lower para-aortic region and in mesentery with minimal uptake. There was no abnormal increased uptake at the rest of the nodal stations, lung, liver, skeletal system, or elsewhere in the body scanned (Figure 2a and b).
Resection of mass was advised in view of solitary lesion and good performance status of patient, as per the oncologist’s opinion. Segmental liver resection was done with cholecystectomy. On cutting serially, circumscribed tumor was identified measuring 7 cm in the greatest dimension. Cut surface was gray-white to brownish with variable sized cystic cavities filled with brownish fluid (Figure 3). Hepatic capsule and base of resection were grossly unremarkable. The gall bladder was unremarkable.
Microscopic examination revealed tumor cells arranged in a follicular pattern, similar of thyroid follicles. Follicles were of varying size and lined by low cuboidal type epithelial cells with pale eosinophilic colloid-like material in the follicles (Figure 4a). Cuboidal cells had round-to-oval nucleus with fine chromatin and inconspicuous nucleoli, resembling follicular neoplasm of the thyroid gland (Figure 4b). No definite desmoplastic stroma or calcification was evident. Lymphovascular emboli were not evident. Surgical resection margins were free of tumor, and adjacent hepatic parenchyma was unremarkable (Figure 5).
IHC results were like biopsy specimen, showing CK 7 and CK 19 positivity with negative TTF-1, PAX8, HepPar1, CK20, CDX2, and synaptophysin (Figure 6a-d).
The final diagnosis of ICC with thyroid-like follicular pattern was offered in view of a single hepatic mass with no other source of primary lesion on PET-CT scan and negative TTF 1 and PAX8 on IHC. The post-operative period was uneventful.
Twelve cycles of adjuvant chemotherapy (injection Gemcitabine) was given after surgery for 6 months. CT scan of the abdomen and pelvis was done at the end of 6 months, showing no evidence of any abnormal enhancing lesion at the operated site or any metastatic lesion elsewhere in the body. After 12 months, during the follow-up period, CA 19-9 was done which was within the normal range (31.73 U/ml). The last follow-up was after 20 months during which CT scan of the abdomen was done suggesting no abnormal enhancing lesion. Clinically patient was doing well at the time of last follow-up.
Discussion
Cholangiocarcinoma is the second most common malignant neoplasm of the liver. On the contrary to hepatocellular carcinoma, cholangiocarcinoma is usually not associated with cirrhosis.
In this case, the microscopic features were closely resembled thyroid follicular neoplasm. It had a predominant follicular pattern. Metastasis to the liver from follicular carcinomas of the thyroid is a wellknown although rare phenomenon [9]. Initially, the morphological differential diagnosis of metastatic well-differentiated thyroid carcinoma was considered, but it was ruled out by the absence of a primary thyroid tumor and lack of immunoreactivity for TTF-1 and PAX8. Other tumors included in the differential diagnosis were hepatocellular carcinoma with acinar or pseudoglandular pattern. However, the lack of reactivity for HepPar-1 and AFP ruled out a hepatocellular carcinoma [10].
Thyroid-like ICC was initially described in 2010 by Fornelli et al. [6]. Another case was subsequently reported by Chable-Montero et al. [7] and Shao-hua Chen et al. [8]. To the best of authors’ knowledge, this case is the first reported in India and second reported in Asia. The clinicopathological findings of all four cases are shown in Table 1.
Malignant tumors having thyroid-like morphological features have been reported in the breast and kidney [11,12]. ICC having thyroid-like follicular pattern is an extremely rare morphological variant that resembles thyroid malignancy. It is not included in the World Health Organization (WHO) classification of ICC [13].
A comprehensive evaluation of case including careful clinical history, physical examination, imaging studies, serum tumor markers, microscopic examination, and IHC workup is warranted to avoid misdiagnosis. Due to the rarity of this morphological variant of ICC, its biologic behavior had to be established, and more cases are required for the study.
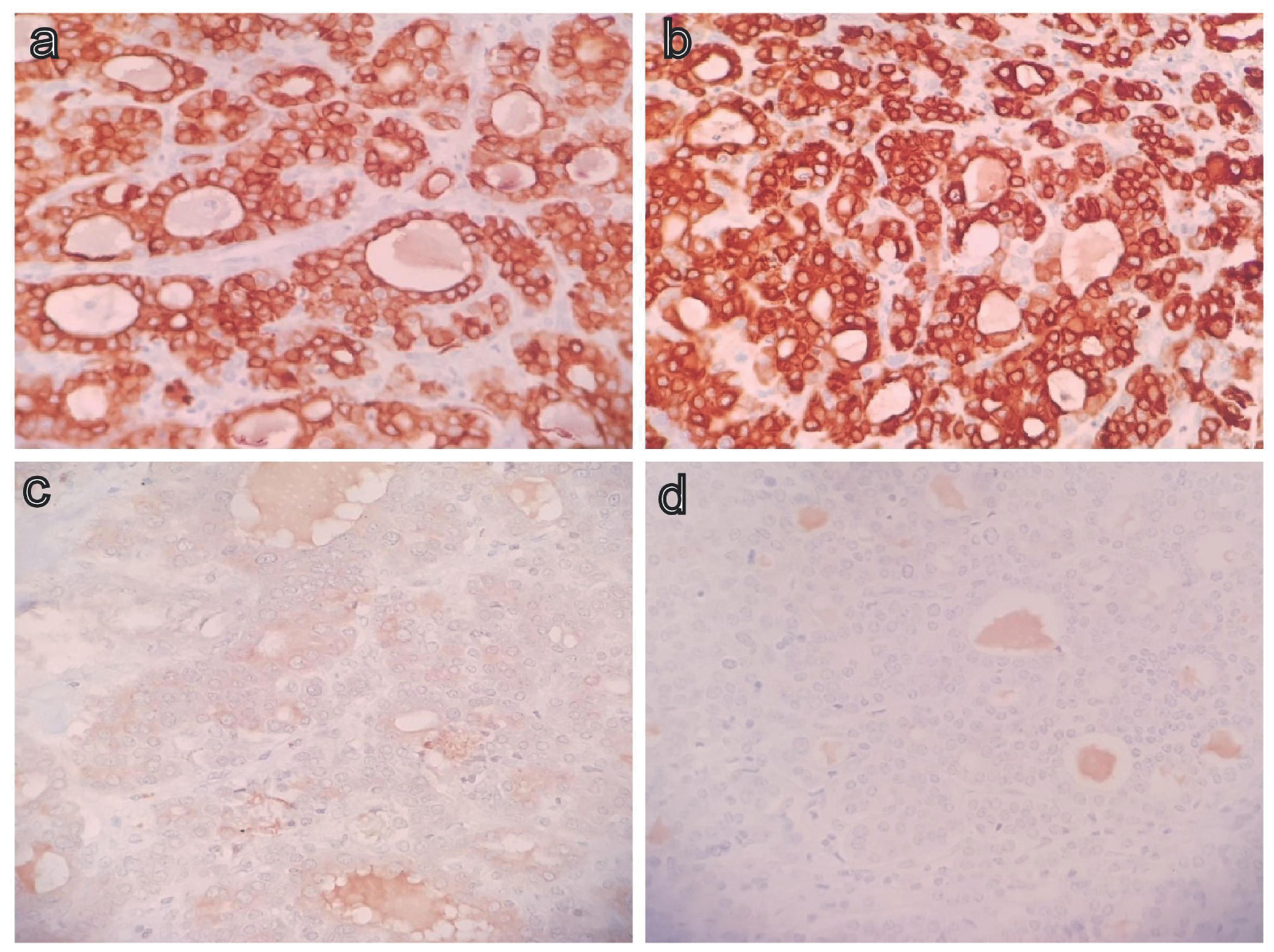
Immunostain positivity for CK 7 (a), CK 19 (b) and negative for TTF 1 (c), PAX8 (d) – 400x.
| FORNELLI ET AL. [6] | CHABLE-MONTERO ET AL. [7] | SHAO-HUA CHEN ET AL. [8] | CURRENT CASE | |
| Age/gender | 52/male | 26/female | 59/male | 42/female |
| Size | 18 cm | 19 cm | 3 cm | 9 cm |
| Gross | Well-circumscribed lesion with cystic cavities | Gray white lesion with cystic and hemorrhagic areas | Gray white solid lesion | Gray white brownish circumscribed lesion with cystic cavities |
| Histology | Follicular pattern of papillary thyroid carcinoma | Mainly follicular with solid, trabecular, and insular pattern | Follicular, papillary, and insular patterns | Follicular pattern |
| Immune phenotype | Positive: CK7, CK19, CAM5.2, CK AE1; Negative: thyroglobulin, TTF-1, CEA, CK20, CD56, synaptophysin, chromog- ranin, hepatic specific antigen | Positive: CK7, CK19, CD138; Negative: thyroglobulin, TTF-1, HepPar 1, glypican-3, AFP, CD56, synaptophysin, chromogranin | Positive: CK7, CK18, CK19, EMA, MUC1, CD10, glypican-3, p53, Ki67, S-100; Negative: thyroglobulin, TTF-1, CD56, synaptophysin, chromogranin, PAX8, CK20, CDX-2, AFP, HepPar 1, CD34 | Positive: CK7, CK19 Negative: HepPar1, AFP, CEA, CK20, CDX2, PAX8, TTF1, Synaptophysin |
| Treatment | Surgery | Surgery and chemotherapy | Surgery | Surgery and chemotherapy |
| Follow-up | 13 months without recur- rence or metastasis | 18 months died with me- tastasis and recurrence | 16 months without recurrence or metastasis | 20 months without recurrence or metastasis |






