INTRODUCTION
Rickettsiae are vector-borne pathogens that affect humans and animals worldwide with an increasing number of members recently identified [1]. Globally, Rickettsia rickettsii in North America, R. conorii conorii in Europe and Africa, R. sibirica in Asia, and R. australis in Australia, have been well-characterized as Rickettsia species frequently resulting in human rickettsioses [2]. According to recent findings, however, various Rickettsia species that have been newly-described from ticks or animals have been shown to cause human illness as well [3–6]. Human rickettsioses are increasingly attributed to Rickettsia pathogens [7] that are much more diverse than historically recognized. From the perspective of clinical practice, the proven Rickettsia species are frequently found to cause atypical clinical symptoms, and even asymptomatic infections in apparently healthy individuals [8–10]. Under such circumstances, it is essential to establish a differential diagnosis of Rickettsia species because an atypical case would likely be underestimated or even incorrectly treated. For example, Rickettsia parkeri was confused with R. rickettsii based on the similarity of symptoms until clinical Western blot techniques were applied [7]. Therefore, close inspection of emerging rickettsioses by applying molecular-based diagnostic techniques is the key to identifying potential rickettsial agents that are capable of causing human diseases and allowing researchers to genetically characterize Rickettsia species prior to culture [11]. We conducted a cross-sectional study among forest rangers who were asymptomatic or had a mild illness after a tick bite, with the expectation that emerging rickettsial agents could be recognized.
METHODS
Human samples collection
The current study was conducted in 2014 among forest rangers in the heavily wooded Da xing’an ling mountainous region along the China-Russia border (Fig 1). The study participants patrolled the wooded areas as part of their routine mission, and were therefore intensely exposed to ticks and at a high risk of tick bites during the season of tick activity despite wearing long-sleeved clothing. As guided by the Center for Diseases Control and Prevention of Forest Rangers, all rangers who had suspected tick bites were expected to seek medical cares within 1 week in the local clinic (the medical detachment of the Inner Mongolia Branch of the General Forest Rangers in Yakeshi). During the hospital visit, blood samples were collected aseptically for detection of Rickettsia agents. All of the samples were stored at −80°C in a refrigerator and transferred in liquid nitrogen before the tests were performed. The samples were transferred to Beijing Institute of Microbiology and Epidemiology for molecular detection. The research protocol had received specific approval from the Institutional Review Board of the Beijing Institute of Microbiology and Epidemiology. Written informed consent was obtained from all participants.
Detection of Rickettsia species
Genomic DNA was extracted from whole blood samples using QIAamp DNA Mini and DNeasy Tissue kits (Qiagen, Germantown, MD, USA), according to the manufacturer’s instructions. Segments of the specific rickettsial 17-kilodalton antigen gene were amplified from genomic DNA using nested PCR with external [17kD1 and 17kD2] and internal primers [17k-5 and 17k-3] [3]. For further confirmation, a nested PCR was performed to amplify the outer membrane protein A (ompA) and B genes (ompB), as previously described [12]. To minimize the risk for contamination, template isolation, PCR setup, and agarose gel electrophoresis were performed in separate rooms. A healthy population without exposure to ticks was used as a negative control. A negative control (distilled water) was also included in each reaction. PCR amplicons were purified and sequenced on a 3730 DNA Sequencer (Applied Biosystems, Foster City, CA, USA) with the same primer sets. All the recruits were also tested for four other tick-borne pathogens that had been previously identified in the same endemic areas, including Borrelia burgdorferi, Babesia protozoan, and Anaplasma phagocytophilum [4]. Only the negative recruitments were further analyzed in our cohort.
Serologic test by indirect immunofluorescence assay
In parallel, serum was tested for the presence of IgM- and IgG-specific antibodies using an indirect immunofluorescence assay (IFA) with Rickettsia heilongjiangensis 045 as the coating antigen. Briefly, cell cultures were fixed on the slides for 10 min in a 1:1 methanol and acetone solution. An IgG antibody titer of 1:64 was considered to be a positive reaction. An acute infection with SFG rickettsiae was confirmed by seroconversion or a four-fold increase in titers of IgG antibodies between acute and convalescent phase serum samples.
Tick samples collection
To determine the possible sources of infection, host-seeking ticks in the forestry area were collected by sweeping flags on vegetation from the same habitats visited by the rangers, the harvested ticks were morphologically identified, then stored and transferred for subsequent molecular detection. Briefly, the frozen ticks were grinded on an automatic sample freezing grinder and genomic DNA was extracted using a QIAamp DNA Mini and DNeasy Tissue kits. Detection of Rickettsia species in ticks was performed by applying the same method as used for humans.
Phylogenetic analysis
Genetic sequences were analyzed with ClusterW software (version 1.83). Sequences obtained in the study were deposited in GenBank. Phylogenetic analyses based on rickettsial sequences were performed using the maximum likelihood method with MEGA software (version 6.0). To assess the reliability of the reconstructed phylogenies, a bootstrap analysis of 1000 replicates was performed.
RESULTS
A total of 159 forest rangers volunteered to participate in the study in 2014. All of the forest rangers were male with a mean (±SD) age of 23.6 ± 3.9 years. Among the 159 forest rangers, 25 (15.7%) had confirmed laboratory results positive for Rickettsia infections, and devoid of any of the three tick-borne pathogens that were simultaneously tested. Only 5 (20%) forest rangers had clinical manifestations following tick bites. One patient reported a headache due to R. heilongjiangensis infection (confirmed by simultaneous detection and sequencing of ompB MH555089, 17kDa MH555090, and ompA MH555091; Fig 2). Headaches, fevers, and myalgias were reported in one forest ranger who was infected with Candidatus Rickettsia tarasevichiae (CRT) that was confirmed by simultaneous detection and sequencing of 17kDa MH549238 and ompA MH549237. The other 3 forest rangers infected by R. raoultii (ompB MH549229, 17kDa MH549230, and ompA MH549231) reported headaches (n=2), and rashes and fevers (n=1). The 5 forest rangers were treated with oral doxycycline (100 mg twice/day) after seeking medical care and recovered 10 days later without any ensuing sequelae.
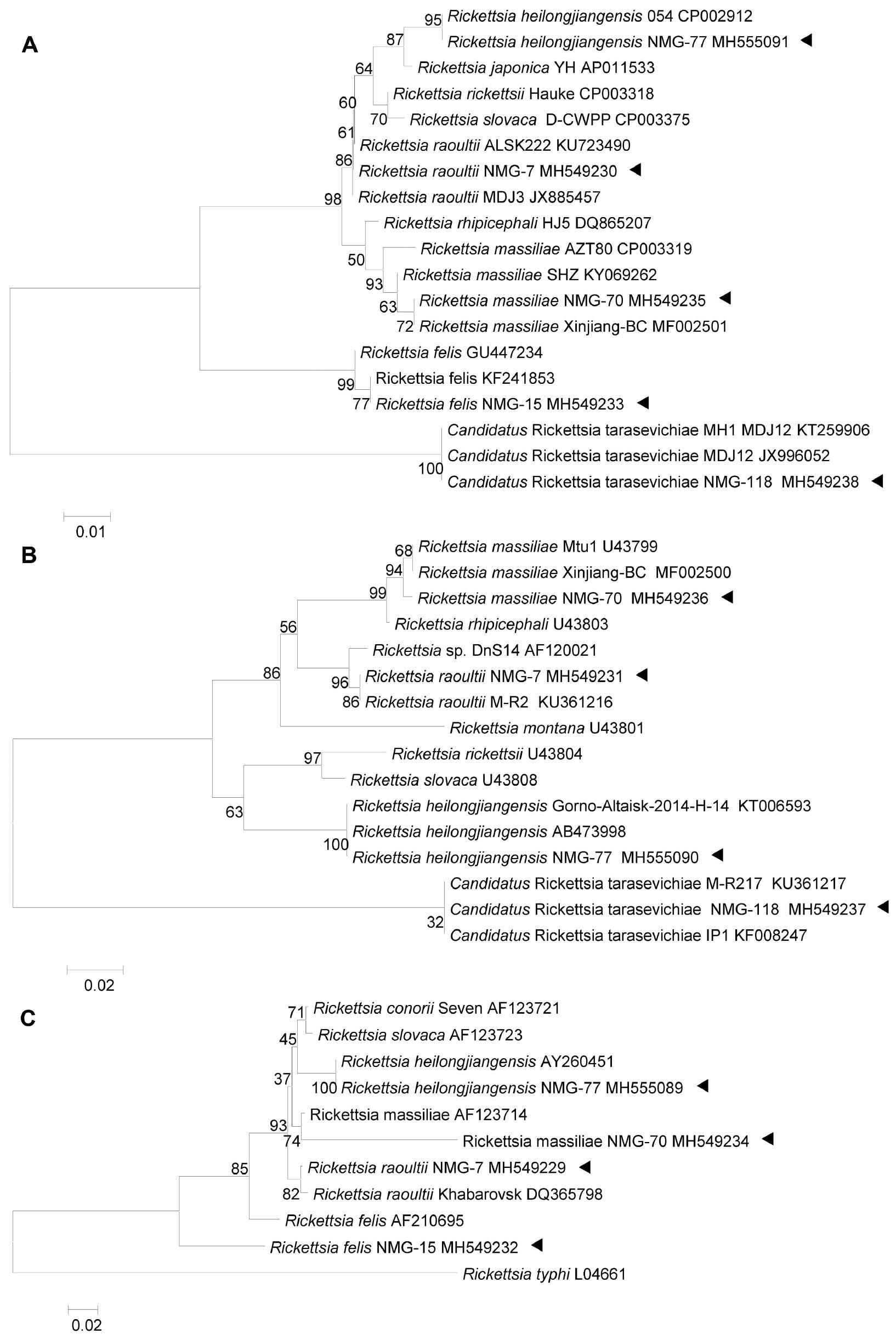
Phylogenetic analysis based on concatenated datasets of 17 KDa, ompA, and ompB partial nucleotide sequences. Phylogenetic trees were constructed based on nucleotide sequences of the 17 KDa (309 bp, panel A), ompA (299 bp, panel B), and ompB (340 bp, panel C) genes using a maximum likelihood model (MEGA, version 6.0; https://www.megasoftware.net). Bootstrap analysis of 1000 replicates was applied to assess the reliability of the reconstructed phylogenies. Scale bars indicate estimated evolutionary distance. The triangle indicates the Rickettsia species detected in the study.
Another 20 asymptomatic infections included R. rauoltii (n=5), CRT (n=13), R. massiliae (ompB MH549234, 17kDa MH549235, and ompA MH549236; n=1), and R. felis (17kD MH549232 and ompB MH549233; n=1 [Fig 2]). Similarly, all of the above gene segments were successfully sequenced, and no gene sense mutations were observed in the corresponding Rickettsia species from the ill forest rangers. None of the 20 asymptomatic individuals developed any clinical manifestations during the follow-up surveys that were conducted within 3 months of sample collection.
All 25 positive serum samples, in addition to PCR amplifications and sequencing analyses, were further submitted for IgM antibody testing using an indirect immunofluorescence assay (IFA). A total of 16 serum samples were shown to contain IgM antibodies against R. heilongjiangensis (Fig 3), of which 7 had titers of 1:64, 7 samples had titers of 1:128, and 2 had titers of 1:256 (including 1 patient who presented with clinical manifestations). The IgM titers of R. massiliae-positive samples reached 1:128 and all 14 participants with CRT infections demonstrated positive reactions (titers ≥1:64) with R. heilongjiangensis coating antigens, including 1 patient who presented with clinical manifestations. No serologic reactions were observed in R. felis or R. raoultii samples, including 3 patients who had clinical manifestations. We further evaluated 8 of the 14 CRT-infected participants 1-3 months after acute infections by collecting second serum samples. A 4-fold increase in the IgG titer was detected in 4 forest rangers and a 2-fold increase of the IgG titer (from 1:64 to 1:128 and 1:128 to 1:256) was noted in another 2 forest rangers, while no significant change was observed in the remaining 2 serum sample pairs.

Representative photograph of immunofluorescence assay results.
Panel A shows the immunofluorescence assay results of a convalescent serum sample obtained from a patient. Panel B shows the immunofluorescence assay results of a negative control sample.
The possible sources of Rickettsia species infections were determined by testing host-seeking ticks that were concurrently captured from the same habitats by sweeping flags on vegetation. A total of 780 ticks (Ixodes persulcatus [n=713], Haemaphysalis concinna [n=47], and Dermacentor silvarum [n=20]) were screened for the presence of Rickettsia species using the same PCR amplifications as used for human infections. CRT was present in I. persulcatus (38.4%; 274/713) and Hae. concinna (6.4%; 3/47). R. raoultii was detected in D. silvarum (30.0%; 6/20) and Hae. Concinna (14.9%; 7/17). R. heilongjiangensis was present in D. silvarum (9.5%; 2/21) and I. persulcatus (0.3%; 2/713). Neither R. massiliae nor R. felis was detected among these ticks.
DISCUSSION
In the current study a series of mild or asymptomatic infections caused by Rickettsia species were determined in a group of forest rangers who reported tick bites in northern China. Multiple rickettsial organisms were shown to be responsible for the infections, including R. heilongjiangensis, CRT, R. rauoltii, R. massiliae, and R. felis. Among the 5 rickettsial species, R. heilongjiangensis was described in northern China and neighboring areas decades ago [13]. R. rauoltii, CRT, and R. felis, all of which are recently emerged rickettsia [5,6,14], were extensively related to human rickettsioses in China. The current study implicated the existence of subclinical infections, therefore the actual incidence and the impact on public health might be underestimated.
As one of the emerging Rickettsia species, R. massiliae was first isolated from Rhipicephalus ticks in Marseille, France [15]. Until recently, only 4 European cases were confirmed with clinical manifestations including fevers, rashes, eschars, neck lymphadenopathy, and acute loss of vision [10,16,17]. No mild or asymptomatic R. massiliae infections have ever been documented. Although only one case was recorded in the current survey, additional investigations are warranted to elucidate the clinical spectrum. R. felis is another emerging SFG rickettsial pathogen; the cat flea, Ctenocephalides felis, is known as a competent vector [18]. The clinical manifestations of R. felis infection resemble murine typhus and dengue, making R. felis infection difficult to diagnose if appropriate laboratory testing is unavailable [8,14,18]. The discovery of one victim with an R. felis infection in our survey corroborates previous reports on R. felis in Chinese populations [14]. Although R. felis was recently isolated from an I. ricinus nymph tick [19], the role of ticks and other vectors in transmitting the agent needs to be determined. CRT, a member of the ancestral R. canadensis group [17], was first described from I. persulcatus ticks collected in the Russian Urals and Siberia in 2003 [20]. The pathogenicity to humans was recently demonstrated in a group of patients in China [4,5]. In addition to I. persulcatus, Dermacentor species might also be involved in the maintenance of CRT [5]. The prevalence of CRT in the ticks sampled in the same habitats suggested the possible roles of Ixodes persulcatus and Dermacentor species in the transmission of CRT to the forest rangers. R. raoultii, the causative agent of Dermacentor-borne necrosis erythema lymphadenopathy or tickborne lymphadenopathy (DEBONEL-TIBOLA), has been frequently recorded in women and children in Europe [21], but results in atypical infections in patients of all ages and both genders in China [6]. The present study confirmed previous evidence that a differential clinical presentation could be associated with R. raoultii infection.
Our previous study revealed that CRT infection results in fevers (96%), malaise (88%), myalgias (57%), cough (25%), and dizziness (14%) in 56 hospitalized patients with a median age of 59 years [4]. Asymptomatic CRT infections in the current study may reflect the much younger age of the forest rangers or because the infections were identified in an early stage after the tick bite. Follow-up for these individuals with asymptomatic CRT infections should be improved in the future.
The current study was subject to a major limitation that only partial fragments of coding genes were detectable, making a detailed and comprehensive genetic alignment impossible. In contrast, the clinical features were milder than previously reported, partially because the subjects were young, male, immunocompetent adults. The low pathogenic characteristics of these Rickettsia agents might also have been responsible. Either way, screening for Rickettsia species in apparently healthy subjects offers a new way to comprehensively acquire the clinical features of these newly identified tick-borne rickettsioses, and suggests the necessity of conducting in-depth investigations in the future.


