INTRODUCTION
Acute diarrheal disease (ADD) or gastroenteritis is a disease caused by bacteria, viruses, and parasites, and is characterized by increased bowel motility, which causes watery or loose stools [1]. ADD is the most common disease globally and the principal cause of death among children <5 years of age [2]. Indeed, most children <5 years of age worldwide have had gastroenteritis [3]. Moreover, the number of children <5 years of age worldwide with infectious diarrhea caused by rotavirus (RV) infection has been estimated to be approximately 258 million [4]. Between 2013 and 2017, RV induced 122,000–215,000 diarrheic child deaths annually [4–6]. RV ranks third among leading pathogens associated with mortality among all causes of death in children <5 years of age [6]. Children in low- and medium-income countries (LMICs) represent the majority of diarrheal deaths compared to high-income countries (HICs) [4]. A worldwide health-related statistic from 2016 revealed that among 10 developing countries, approximately 100/100,000 children die before reaching 5 years of age, representing the highest RV diarrheic deaths [5]. Currently, live attenuated oral RVs are used as the main strategy to control RV diseases, especially in countries with high mortality rates [7]. World Health Organization (WHO) approved two RV vaccines (RotaTeq [RV5] and Rotarix [RV1]) in 2008 and 2009, respectively, that are the most extensively used vaccines worldwide for the prevention of RV infection [7]. Although the WHO recommended the inclusion of RV vaccines into all national immunization programs, more than 100 countries, including Egypt, have not introduced the vaccines into their compulsory immunization program [8]. Thus, it is important to continuously survey rotavirus genotypes to verify if the oral vaccines provide full protection against the common RV genotypes infecting Egyptian children. This information is crucial to enhance vaccine development, detect emerging genotypes, and aid in the evaluation of vaccine efficacy and changes in strain diversity after vaccines have been introduced.
Gastroenteritis in animals is caused mainly by RV that was isolated from numerous species of domesticated and wild mammals [9,10], as well as birds [11]. Subsequently, these infections significantly led to economic losses in different livestock (cattle, swine, and horses) due to the weight loss of affected animals and the cost of treatment. There is increasing proof of interspecies transmission and reassortment between human and animal RVs. Some species, such as cattle, pigs, dogs, and cats, have already been shown to share the RV genetic diversity detected in humans [12]. Neonatal calf diarrhea is one of the most common diseases in the dairy industry, and is characterized by high morbidity and mortality [13]. Group A RVs are the main etiologic agents for calf diarrhea, which cause 5%–20% of calf losses [14].
In 2010 the WHO estimated that 1.8 billion people drink unsafe water and 1.2 billion people drink contaminated water. In fact, 700,000 diarrheal deaths globally are attributable to contaminated water each year [15]. Urban wastewater discharged into surface water can act as a source of environmental viral contamination. Contamination of the environment can also occur from the reuse of wastewater for agriculture or industrial purposes. Enteric viruses are major causes of gastroenteritis [16] and frequently replicate in the gastrointestinal tract. Enteric viruses are excreted in human feces, in which an infected person can shed up to 105–1013 viral particles/g [17,18]. Enteric viruses have been found not only in wastewater, but also in rivers, recreational water, and seawater, as well as ground water and even treated drinking water [19]. Symptomatic and asymptomatic persons shed a huge number of viruses into the sanitary network each day. Therefore, wastewater is one of the major concentrated sources of human enteric viruses in the environment. Moreover, if the sanitary network is broken, or untreated or partially treated wastewater is released directly into the environment, pollution of other environmental water sources (groundwater, rivers, and pond water) may occur [20]. Sporadic cases and outbreaks of gastroenteritis have been detected in association with RV, norovirus, astrovirus, and adenovirus, which are also important agents of water-related diseases [17].
Herein, the epidemiology of RV disease is highlighted and the prevalence of G and P RV serotypes is analyzed on the basis of data collected from PubMed or other local databases focusing on children with RV-related diarrhea, calves with diarrhea, and environmental samples in Egypt between 1992 and 2022. The possibility of interspecies transmission and reassortment of RV between human and animals are discussed.
ROTAVIRUS STRUCTURE
RV belongs to the Reoviridae family and is formed from a triple-layered particle (TLP) that consists of three types of particles (double-shelled, single-shelled, and core) arranged in concentric rings around the genome [21]. The TLP is the infectious form of the virus [22]. The diameters of the double-shelled, single-shelled, and core particles are 76.5 nm, 70.5 nm, and 50 nm, respectively. RV is comprised of a double-stranded RNA (dsRNA) genome that is composed of 11 segments. Each segment encodes 1 of 6 structural viral proteins (VP1-4, VP6, and VP7) or 5-6 non-structural proteins (NSP1-5/6) [1,23]. The viral capsid proteins (VPs) are the major antigenic proteins of the RVs [24], while the NSPs are produced during infection to aid viral replication and pathogenesis [25].
ROTAVIRUS GROUPS
The RV gender includes viruses that only infect vertebrates (birds and mammals) [1]. The RVs have a common antigen (protein VP6), which forms the middle layer [26] (group antigen) [1]. RV can be classified based on VP6 into 10 groups (RVA-RVJ) according to the International Committee of Taxonomy of Viruses (ICTVs) [1,25,26]. RVK and RVL were also reported, but not included in the ICTVs [27]. RVA, RVB, RVC, and RVH infect humans and animals. Group B rotavirus has been identified in humans and some animal species (cattle, sheep, pigs, dogs, and rats). Group C RV affects pigs, cattle, humans, ferrets, and dogs [1]. RVH was first identified in humans in China and Bangladesh, more recently in pigs in Japan and Brazil [28,29]. RVD, RVE, RVF, and RVG have only been identified in animals [1,25]. RVD, RVF, and RVG only infect birds [1,30]. RVE has only been found in pigs [12]. Recently, RVI and RVJ were detected in dogs and bats, respectively [26,31].
CLASSIFICATION OF GROUP A ROTAVIRUS
RV classification is based on a binary classification system according to immunologic reactions and the structure of VP7 and VP4 protein genes into glycoprotein (G) and protease-sensitive (P) genotypes, respectively, which independently stimulate neutralizing antibody production [24,25]. Currently, 42 G-types and 58 P-types have been described according to the RV Classification Working Group based on global investigation reports in both humans and animals [32]. More recently, the whole genome or 11-gene typing system replaced the binary strain typing system to ascribe genotypes to each gene (Gx-P[x]-Ix-Rx-Cx-Mx-Ax-Nx-Tx-Ex-Hx), which codes for VP7-VP4-VP6-VP1-VP2-VP3-NSP1-NSP2-NSP3-NSP4-NSP5/6, respectively [33]. The number of genotypes and the function of the encoded proteins are shown in Table 1 [34].
Number of genotypes ascribed to each gene segment (updated) and the biological functions of the encoded proteins [33,34].
| Genome segment | Size (bp) | Number of genotypes | Genotype denotation | Protein product | Type of protein/location in the virion | Function |
|---|---|---|---|---|---|---|
| 1 | 3302 | 22 | R | VP1 | Structural, inner capsid | - RdRp - ssRNA binding |
| 2 | 2687 | 20 | C | VP2 | Structural, core | - Houses RNA genome |
| 3 | 2592 | 20 | M | VP3 | Structural, inner capsid | Guanyltransferase Methyltransferase ssRNA binding |
| 4 | 2362 | 51 | P | VP4 | Structural, outer capsid | - Receptor binding protein - Infectivity enhancement through trypsin cleavage |
| 5 | 1356 | 26 | I | VP6 | Structural, middle capsid | Serologic grouping and subgrouping antigen |
| 6 | 1062 | 36 | G | VP7 | Structural, outer capsid | - Neutralization antigen - Basis of binary classification |
| 7 | 1581 | 31 | A | NSP1 | Non-structural | - Host interferon antagonist - Anti-apoptosis |
| 8 | 1059 | 22 | N | NSP2 | Non-structural | - Helicase - NTPase - NDPK - RBP |
| 9 | 1074 | 22 | T | NSP3 | Non-structural | - Competition with host PABP for self-4G1 binding - Translation enhancer |
| 10 | 751 | 27 | E | NSP4 | Non-structural | - Enterotoxin - Transmembrane gp |
| 11 | 666 | 22 | H | NSP5 | Non-structural | - Phosphoprotein |
| NSP6 | Non-structural | - ssRNA and dsRNA binding |
Note: RdRp = RNA-dependent RNA polymerase; PABP = poly (A) binding protein; RBP = RNA binding protein; NDPK = nucleoside diphosphate kinase.
ROTAVIRUS DETECTION AND STRAIN CHARACTERIZATION
Fresh stool samples or rectal swabs are the main samples used to detect RV infection by confirming the presence of the RV, virus-specific antigen, or RNA [35,36]. The laboratory diagnosis of RV includes several techniques, such as electron microscopy (EM) [37], which recognizes and identifies the virus depending on morphologic characteristics; however, this technique is expensive, needs well-trained workers, and a large number of persons for the routine diagnosis of RV in a large number of specimens. Also, many commercially-available antigen detection kits, such as ELISA, latex agglutination, or immunochromatography, are used to diagnose RV. The latex agglutination technique is simple, quick, and easy to perform without the need of complicated equipment. Therefore, the latex agglutination technique is useful in disease outbreak detection [38]. ELISA is the most widely used antigen detection method because of high sensitivity, specificity, and ability to test large number of samples in 96-well plates [35,36]. Virus isolation and growth in cell lines is a useful technique to confirm virus viability and enhance molecular detection of the virus, especially if the virus is in very low concentration in environmental or clinical samples [39–41]. Although viral isolation from cell lines is highly sensitive, this method is expensive, difficult, easily contaminated, and often not required for routine clinical diagnosis. Different types of polymerase chain reactions (PCRs), such as reverse transcription (RT-PCR), quantitative (q)PCR, and real-time PCR, detect RNA in clinical or environmental samples and are more sensitive techniques than other antigen detection methods [35,42,43]. For genotyping of circulating RV strains, VP4 and VP7 sequences, and other genome segments are required. More recently, the RV Classification Working Groups recommend whole genome sequencing for complete characterization of the RV genome and recognition of unusual genotypes [44].
MODE OF TRANSMISSION
RV is mainly transmitted via the fecal-oral route [45]. As shown in Fig 1, the spread of human feces is often enhanced by environmental factors, cush as fingers, foods, fluids, and fomites through interactions between humans or animals with the environment [46]. The spread of RV is common among children; moreover, transmission to close contacts is highly likely from infected children. RV has the ability of interspecies and cross-species transmission; reassortments occur, which are the main mechanisms that cause diversity of RVs and the emergence of new strains [47].
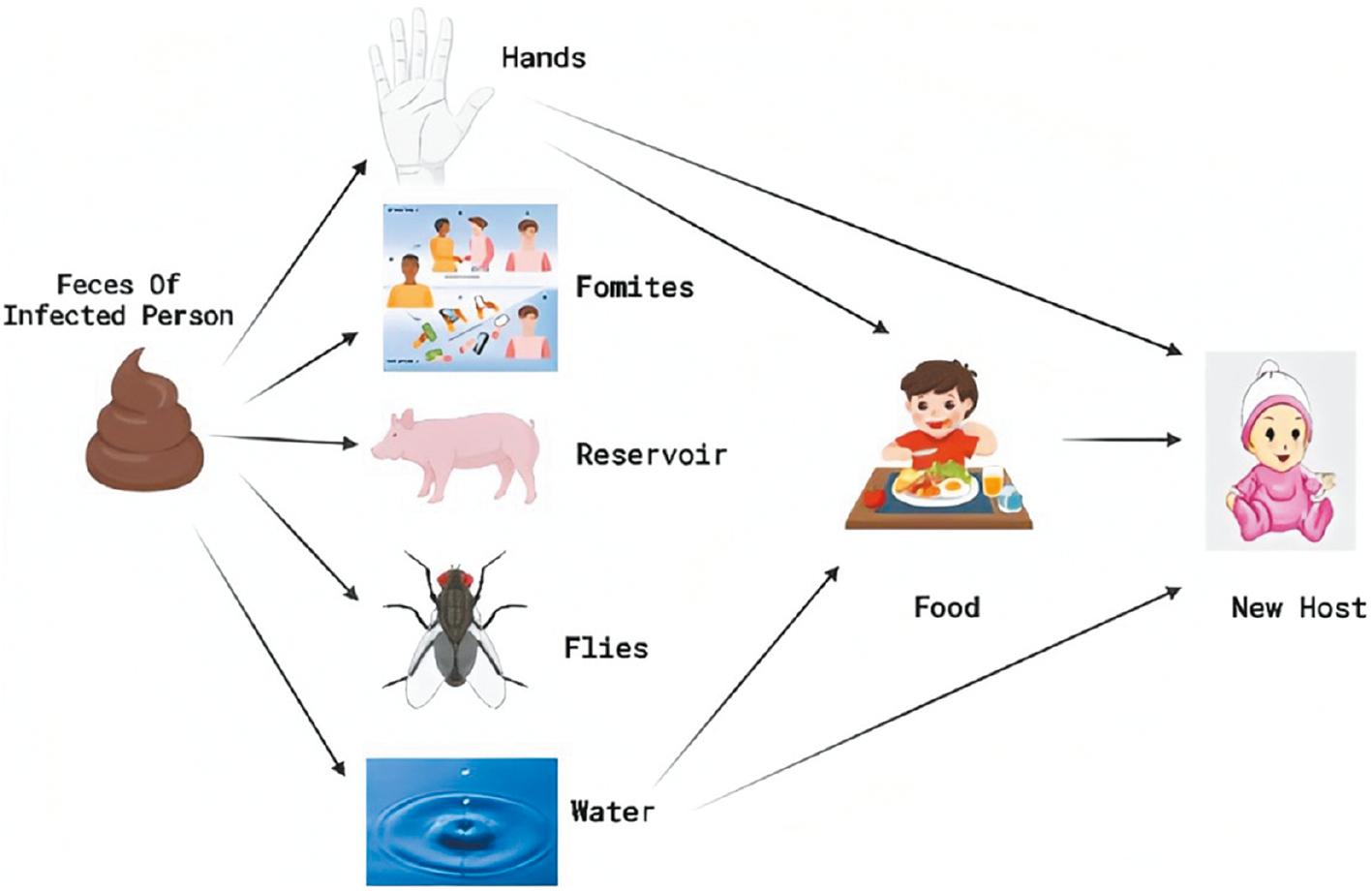
The figure shows the mode of rotavirus transmission. The primary mode of transmission is the transfer of the virus in the stool of one child-to-the mouth of another child (fecal-oral route). Other possible means of transmission are contaminated food, contaminated water, and fomites. The vertical lines (black) show barriers against transmission, i.e., toilet barrier, clean water barrier, hand washing, and proper hygiene barrier [21].
CLINICAL FEATURES
Neonates (<1 month of age) often have asymptomatic or mildly symptomatic RV infection due to protection provided by maternal antibodies transferred via the placenta and breast milk [48]. The clinical symptoms of RV infection include the following: no symptoms-to-mild symptoms; and watery diarrhea of short duration-to-severe diarrhea with vomiting and fever that can cause rapid dehydration with shock, electrolyte imbalance, and death [24]. The incubation period of RV is 18–36 h and is followed by the onset of acute fever and vomiting [49]. In children, more than one episode of RV infection may occur due to the inability of the natural infection or the vaccine to provide full protection against future infections. Remarkably, the primary infection induces more severe signs than recurrent infections [50].
RV diarrhea in calves is an acute infection with a very short incubation period of 12–24 h that ranges from 18–96 h. RV in calves is characterized by high morbidity, although infection is usually mild and self-limiting. The clinical symptoms in calves are affected by host age, host immune status, host nutrition status, environmental stress (such as housing, overcrowding or weather conditions), variation in virulence among RV strains, and occurrence of mixed infections. The clinical features of RV infection in calves involve fluid loss and metabolic acidemia, anorexia, profuse watery diarrhea, and different degrees of systemic dehydration [51]. In severe cases, death occurs as a consequence of electrolyte imbalances, dehydration, and cardiac arrest [52].
DISTRIBUTION OF GROUP A ROTAVIRUS STRAINS IN EGYPTIAN CHILDREN
Genotyping of RVA strains in epidemiologic investigations is determined by G and P types. Due to the segmented characteristic of the RVA genome, the genes that encode for VP7 and VP4 can segregate in an independent manner, leading to a large diversity of strains. Over the last 30 years, RV epidemiologic studies have been conducted in both urban and rural areas in Egypt. Between 1992 and 2008, a total of 5804 samples from children were subjected to RV detection and genotyping, of which 671 (11.5%) were positive. G1 was the most predominant genotype, followed by G2, then G3 and G9; G4, G8, and G12 were also detected during this period. Between 2008 and 2022, a total of 2552 samples were subjected to RV detection and genotyping, of which 829 (32.5%) were positive. G1 remained the predominant genotype, followed by G3, then G9, G2, G4, G8, and G12. These studies were carried out on patients with acute diarrhea from outpatient clinic visits or hospitalizations. These studies showed an average RV prevalence of 15%–100%. Data are shown in Figs 2 and 3, and Table 2.
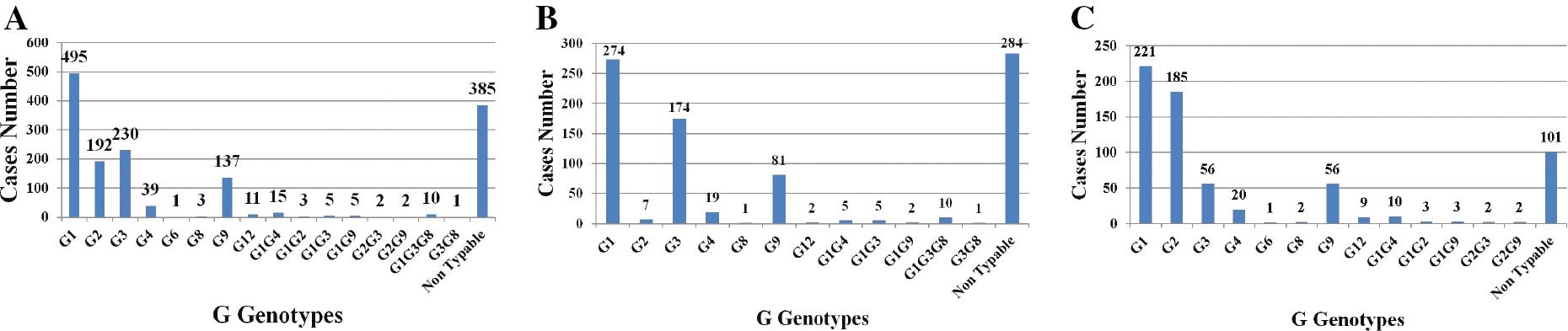
A; Distribution of G genotypes in samples (n=1500) collected from children between 1992 and 2022 in Egypt. B; G genotypes in children between 1992 and 2008 (n=671) in Egypt. C; G genotypes in children between 2008 and 2022 (n=829) in Egypt.
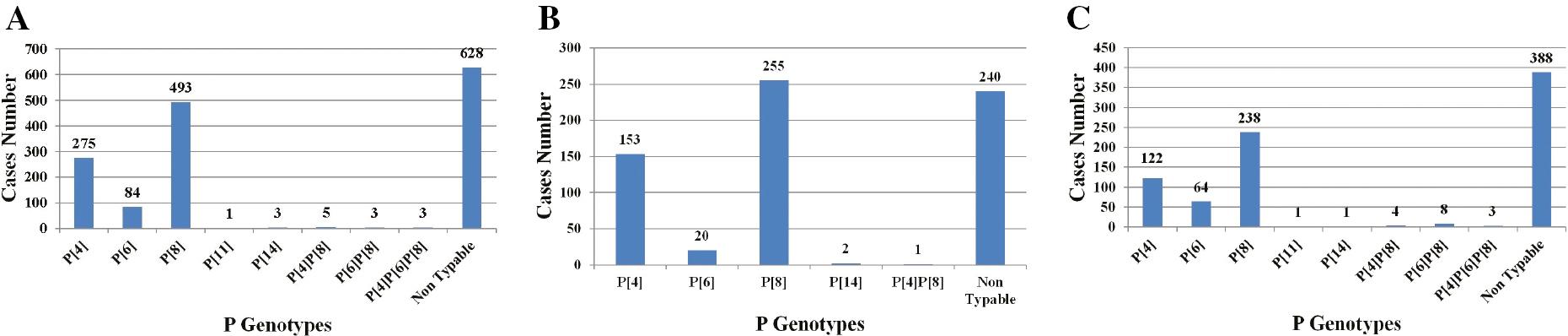
A; Distribution of P genotypes in samples (n=1500) collected from children between 1992 and 2022 in Egypt. B; P genotypes in children between 1992 and 2008 (n=671) in Egypt. C; P genotypes in children between 2009 and 2022 (n=829) in Egypt.
Rotavirus genotypes among Egyptian children with acute gastroenteritis.
| Area of study | Year of study | Number of stool samples tested | Age (years) | Percent positive with rotavirus | Predominant G genotype | Predominant P genotype | Reference |
|---|---|---|---|---|---|---|---|
| Cairo University Children’s Hospital | August 1992 and October 1993 | 180 | <1 year | 35.60% | G1 and G4 | Not typed | [53] |
| Abu Homos, Egypt | February 11, 1995–February 10, 1996 | 178 | <3 years | 25.80% | G2 | P[4] | [54] |
| Abu Homos, Egypt | 1995-1996 | 2 | 17 and 30 months | 100% | G8 | P[14] | [55] |
| Abu homos District Hospital and Banha Fever University | May 2000–May 2002 | 1026 | <5 years | 25.2% | G1 | P[8] | [56] |
| Abu Homos District Hospital | 2004 | 1 | <5 years | 100% | G6 | P[14] | [57] |
| Abu Homos, Egypt | January 2004–April 2007 | 4001 | Birth-2 years | 5.60% | G2 | Not typed | [58] |
| Assiut Pediatric University Hospital | December 2005–April 2006 | 88 | <3 years | 50% | G3 | Not typed | [59] |
| Alexandria University Children's Hospital | January 2006–December 2006 | 100 | <5 years | 33% | G4 | Not typed | [60] |
| Tabarak Hospital Cairo, dispensaries in Giza | March 2006–February 2007 | 230 | 1 month-18 years | 33% | G2 | P[4] | [61] |
| Abu-El Rish hospitals, Cairo, Egypt | February 2009–January 2010 | 100 | <4 years | 19% | G1 | P[4] and P[8] | [62] |
| Cairo (El-Demerdash Hospital) [200/450] Fayoum (Fayoum General Hospital) [150/450] Sharkia Governorate (Belbes General Hospital) [100/450] | May 2009–April 2010 | 450 | Infants and children <5 years | 35% | G1 | Not typed | [63] |
| Mansoura University Children’s Hospital | September 2010–February 2012 | 92 | <3 years | 48.90% | G1 | P[8] | [64] |
| Cairo University Children Hospital, Al-Saff Children Clinic | August 2011–August 2012 | 197 | 2 years of age with acute diarrhea | 39.10% | G3 | P[8] | [65] |
| Abu-El Rish Hospital, Cairo, Egypt | May 2015–April 2016 | 198 stool specimens | <5 years | 28.30% | G3 | Not typed | [66] |
| Abou El-reesh Children’s Hospital of Pediatrics in Cairo | June 2015–May 2016 | 119 fecal diarrhea | <5 years | 31% | G1 | P[8] | [67] |
| Abo El-Reech Hospital, in Greater Cairo, Egypt | October 2015–September 2017 | 1026 stool specimens | <5 years | 24.37% | G1 | P[4] | [68] |
| Hospitals and Special Clinics from Qalubia and Cairo Governorates, Egypt | April 2018–June 2019 | 70 stool specimens | Not mentioned | 35.70% | G1 | P[8] | [69] |
| Abo El-Reesh and El-Demerdash | December 2018–April 2020 | 230 | Birth-5 years | 14.80% | G1 | Not typed | [70] |
| Children Hospital, Zagazig University | January 2019–January 2020 | 140 stool specimens | 1 month-5 years | 91.40% | G3 | P[8] | [71] |
G1–G4 genotypes
All 4 globally-important RV G types (G1–G4) of epidemiologic significance have been detected in Egypt. Each genotype was predominant over a specific time period. The first study genotyping human RV, which was conducted in Egypt in 1992, revealed that G1 and G4 were equally predominant genotypes [53]; however, other reports have revealed that the G1 genotype was mostly predominant in 2002 [56], between 2009 and 2012 [62–64], 2015–2017 [67,68], and 2018–2020 [69,70]. The G2 genotype was the most predominant genotype during the 1995–1996, 2004–2007, and 2006–2007 seasons [54,58,61]. Other studies indicated that the G3 genotype was predominant during the 2005–2006, 2011–2012, 2015–2016, and 2019–2020 seasons [59,65,66,71]. G4 was the predominant strain during the 1992–1993 season [53] and in 2006 [60].
P[8], P[4], and P[6] genotypes
P[8], P[4], and P[6] are the major P genotypes, accounting for nearly 99% of all human RV infections occurring in Egypt. P[8] was the most prevalent genotype in 2002 and between 2009 and the present studies reported that P[8] represented >50% of all P genotypes detected in many regions of Egypt during the study period [56,62,64,65,67,69–71]; P[4] and P[6] were the second and third most common P genotypes identified, respectively [56,61,64,66–69]. Other investigations reported that the P[4] genotype was predominant during February 1995 through February 1996 in Abu Homos district, March 2006 through February 2007 in Cairo and Giza governorates, Feb. 2009 to Jan. 2010 and Oct. 2015 to Sep. 2017 in Cairo [54,61,62,68].
CLINICAL SIGNIFICANCE OF UNUSUAL G GENOTYPES IN EGYPT
G9 genotype
G9 is known to be the fifth most common genotype, after the G1–G4 genotypes, currently circulating in the human population. The first G9 rotavirus, WI61, was identified in children in the USA in 1983 [72]. Subsequently, G9 strains have been commonly described as the causative agents of diarrhea among children and have been identified in several countries as one of the most widespread and emerging genotypes [73]. In Egypt, the G9 strain was first identified during the 2000–2001 rotavirus season [60]. Subsequently, G9 strains have been identified during successive seasons across the country [56,58,61–69].
G12 genotype
G12 was first detected in1987 in the Philippines and was designated L26. The G12 genotype was first identified in Egypt during the 2006–2007 season [61]. Another study detected the G12 genotype during the 2011–2012 season [65].
G10 genotype
Research analyses showed that the G10 RV strain has seldom been identified in Egypt. Only one G10 strain has been detected in Egypt, and this occurred during the 2015–2016 season [66]; however, more studies are warranted to determine the importance and distribution of this RV strain in the Egyptian population.
HUMAN ROTAVIRUS MIXED INFECTIONS
Mixed and multiple RV infections have been recorded in Egypt. The first RV mixed infection involving G1 and G2 was recorded in 1992 [54]. Another study [58] reported the first co-infection involving G2 and G3, and G2 and G9. Amer et al. [60] detected the first mixed RV infection involving G1 and G9. A mixed infection involving G1and G4 was also recorded during the 2009–2010 season [63]. A previous study described a mixed infection involving G1 and G3 [65]. Another study detected mixed infections involving G1 and G9, and G1 and G4 [64]. Multiple infections involving G1/G3/G8, and G3/G8 were described for the first time in Egypt during the 2015–2016 season [66].
SEASONAL VARIATION OF ROTAVIRUS IN HUMANS
RV infections occur in Egypt throughout the year. Most studies have shown that peak infections mainly occur in the winter [60–63,65,68,70]. Saudy et al. [64] and Ahmed et al. [58] reported peak RV infections in autumn. Allayeh et al. [67] and Shaheen et al [66] showed that peak RV infections occurred in the spring.
DISTRIBUTION OF ROTAVIRUS STRAINS IN ANIMALS
G6, G10, and G8 combined with P[5], P[11], and P[1] are considered to be important bovine RV-group A genotypes [74]. Bovine RV was first detected in Egypt in 1981 [75]. Since that time, numerous studies have been conducted that detected RV in cattle calves [76–82]. Only two studies genotyped circulating bovine RV strains in Egypt, in which G6 was the predominant genotype followed by G10; however, P[5] was the predominant serotype followed by P[11] with the detection of only one mixed infection involving P[5] and P[11] [83,84]. Hemida [85] confirmed the first group D avian RV in Egypt. Data are shown in Figs 4 and 5 and Table 3.
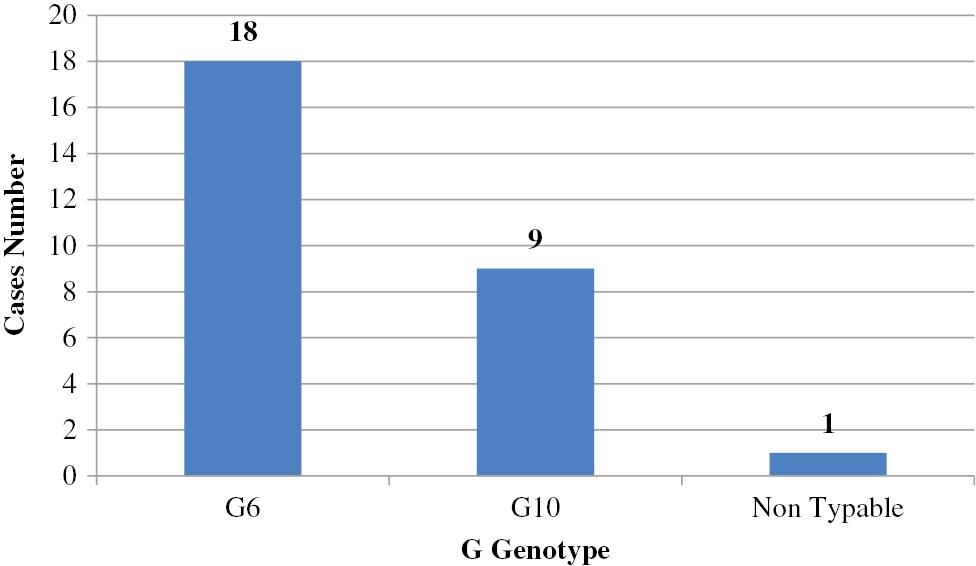
Prevalence of G genotypes in calves (n=28) in Egypt. G6 was the predominant genotype, followed by G10.
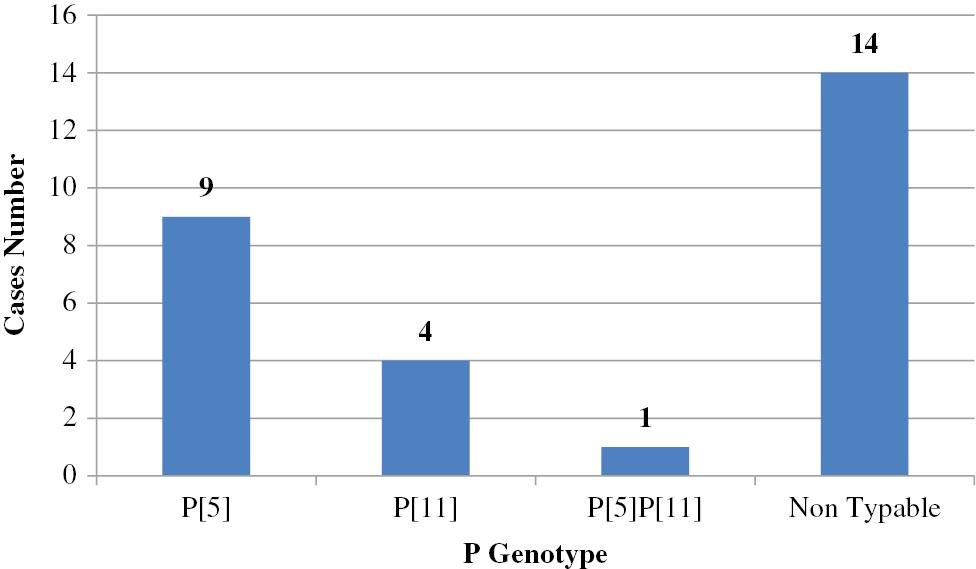
Prevalence of P genotypes in calves (n=28) in Egypt. P[5] was the predominant serotype, followed by P[11].
Rotavirus genotypes among Egyptian calves and camels with acute gastroenteritis.
| Area of study | Year of sample collection | Number of stool samples tested | Age (years) | Percent positive with rotavirus | Predominant G genotype | Predominant P genotype | Reference |
|---|---|---|---|---|---|---|---|
| Alexandria and Ismalia | 2004–2005 | 85 | Camel calves 2 weeks–4 months old | 9.50% | G10 | Not typed | [86] |
| Sharkia and Cairo | Early 2015 | 25 | Calves 3 weeks–10 months old | 48% | G6 | P[11] | [83] |
| Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University | January 2018–November 2019 | 16 | Calves 3–60 days old | 100% | G6 | P[5] | [84] |
SEASONAL VARIATION OF ROTAVIRUS IN ANIMALS
The peak RV infection in animals was detected in the winter [80,84]. RV infections peak during the cold months. This finding may be due to an increased capability to survive at low relative humidity and temperature. Moreover, during the spring and summer the titer of immunoglobulins, such as IgA, IgM, and IgG in colostrum, which provide protection against infections in calves increases while immunoglobulin titers decrease in the autumn and winter [87,88].
DISTRIBUTION OF ROTAVIRUS STRAINS IN ENVIRONMENTAL SAMPLES
Many researchers have investigated the prevalence of human RV VP4 and VP7 genotypes in environmental water samples; data are shown in Figs 6 and 7 and Table 4.
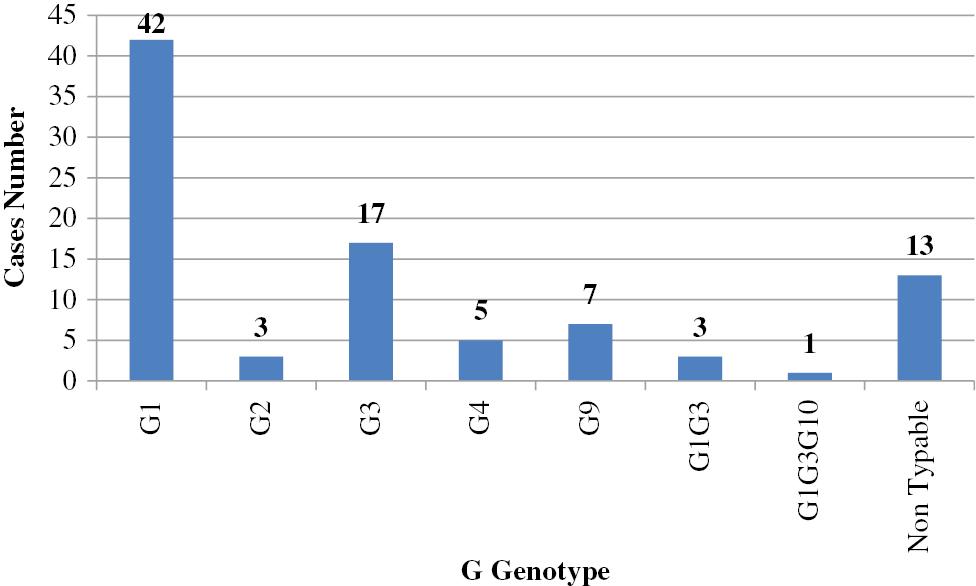
Prevalence of G genotypes in sewage samples (n=91) in Egypt. G1 was the most predominant genotype, followed by G3, then G9, G2, and G4.
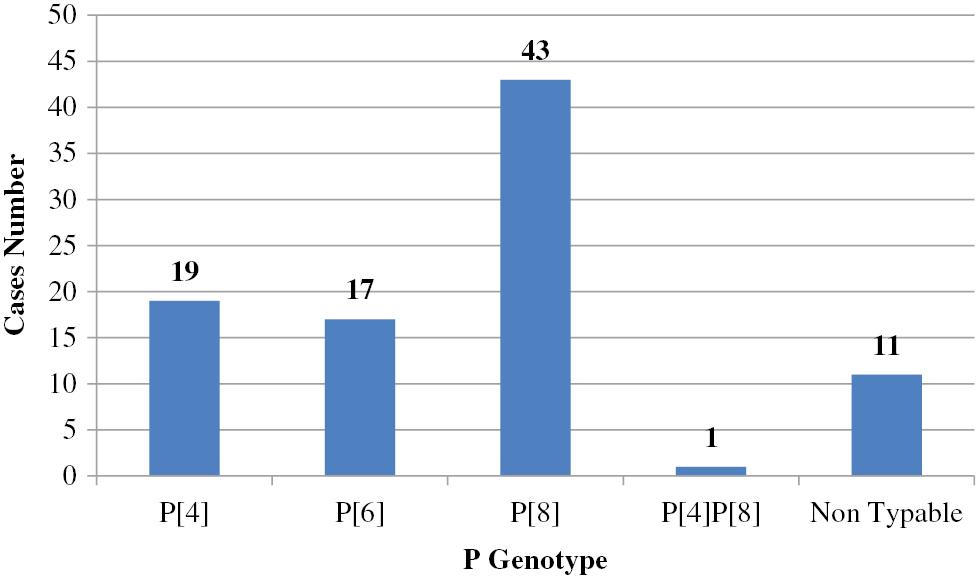
Prevalence of P genotypes in sewage (n=91) in Egypt. P[8] was the predominant genotype, followed by P[4], then P[6].
Rotavirus genotypes in environmental samples.
| Area of study | Year of sample collection | Number of sewage samples | Percent positive with rotavirus | Predominant G genotype | Predominant P genotype | Reference |
|---|---|---|---|---|---|---|
| Three sewage treatment plants (Balaks, Zenin, and El Berka) in Cairo, Egypt | November 1998–October 1999 | 35 sewage samples | 85.70% | G1 | P[8] | [89] |
| Two waste water treatment plants (WWTPs) located at Zenin and El-berka in Greater Cairo | April 2006–February 2007 | 72 sewage samples | 8.30% | G2 | P[8] | [90] |
| Zenin wastewater treatment plant (WWTP) in Cairo, Egypt | June 2015–August 2017 | - 27 raw sewage - 27 treated sewage - 27 sewage sludge | - 29.9% raw sewage - 7.4% treated sewage - 18.5% sewage sludge samples | G3 | P[8] | [91] |
| Inlets of El-Gabal El-Asfar and Zenin wastewater treatment plants (WWTPs) | October 2015–March 2017 | 24 raw sewage samples | 100% | G1 | P[4] | [92] |
| Wastewater treatment plant (WWTP) in Sharkeya Governorate, Egypt | July 2016–June 2017 | 72 water samples were collected from inlets (n = 36) and outlets (n = 36) | - 23.6% raw sewage - 16.6% treated wastewater | G1 | P[8] | [93] |
| Public café, restaurants and homes found in five separate cities: Cairo, Giza, Helwan, Qalyubia, and Faiyum | December 2016–November 2017 | 180 tap water samples | 15.60% | G1 | P[8] | [94] |
| The Nile water stream passing through Giza | June 2016–May 2017 | 96 water samples | 18.75% | G1 | P[8] | [95] |
Before 2000, G1 was the predominant genotype detected in sewage water [89]. Since that time, two surveillance studies indicated that the G1 genotype was predominant during the 2015–2017 seasons [92,93]. The G2 genotype was the most predominant genotype during the 2006–2007 season [90]; however, another study concluded that the G3 genotype was the predominant genotype [91]. G9 was first detected in sewage samples in 1998 [89], and subsequently detected in other studies [90,91]. G4, G10, and G12 genotypes were also detected in sewage water [89–92]. During the 2016–2017 season, RV A was detected in tap water in which G1 constituted >50% of all collected cases, followed by the G2, G4, and G9 genotypes [94]. Another study investigated the presence of RV in Nile water [95], and revealed that G1 was the predominant genotype, followed by G2, then G3 and G9. Several studies have confirmed mixed infections of different RV genotypes in sewage water [89,91] and in the Nile River [95].
With respect to the VP4 genotypes, P[8] was the predominant genotype detected in sewage water throughout the time period of this review [89–91,93]; however, P[4] was the predominant genotype in only one surveillance study [92]. P[6] was also detected in sewage water [89,91,93]. P[8] constituted the predominant genotype of all tap water samples collected [94]. Samples from the Nile River showed that P[8] was the dominant genotype, followed by P[4], then P[6] [95].
SEASONAL VARIATION OF GROUP A ROTAVIRUS IN ENVIRONMENTAL SAMPLES
RV in raw sewage was shown to peak during the winter season [91,93–95]. The predominance of viral infections during the winter months, which refers to the probable transmission of viral gastroenteritis via a respiratory route, is not completely understood; however, some studies have shown increased virus stability, such as astrovirus, poliovirus, and hepatitis A virus, in the environment at low temperatures [96,97], and therefore higher viral titers in sewage.
DETECTION OF ROTAVIRUS IN ANIMAL PRODUCTS
Enteric viruses are major causes of gastroenteritis foodborne outbreaks. Following infection, viruses replicate in the gastrointestinal tract, then shed in human feces. Enteric viruses are transmitted via the fecal–oral route and ingestion of contaminated foods. In the US, viruses are responsible for 35% and 11% of total hospitalizations and death cases associated with foodborne illnesses, respectively [98]. Some researchers in Egypt have investigated the presence of RV in animal products. In a surveillance investigation involving 2400 Egyptian meat and dairy products conducted from January to December 2007, hepatitis A virus and human RV were detected in 5.33% and 6.75%, respectively [99]. In contrast, another study detected bovine RV by ELISA in raw milk and milk products (cheese and yoghurt) during the 2011–2012 season [100].
INTERSPECIES TRANSMISSION AND ZOONOTIC POSSIBILITY
RVs have a broad host range, and can infect humans and various animal species. As mentioned before, there are antigenic similarities between human and animal RV strains. Hence, whether or not animals can act as a source of RV infection for humans needs clarification. Another theory suggests that upon certain conditions, animal RVs can definitely infect humans and induce disease. The segmented nature of the genome allows viruses, such as influenza virus and RVs, to form new strains via reassortment. This reassortment can occur during viral replication and packaging as a result of genome segment exchange between two different RV strains infecting the same cell [101]. Theoretically, the 11 genome segments of the parental virus strains can reassort into 2048 [101,102] probable genome constellations if reassortment is random.
Holmes et al. [55] reported the detection and isolation of the first G8P[14] RV from stool specimens obtained from two Egyptian children. These two strains (EGY1850 and EGY2295), shared a high level of homology of VP7, and with the VP7 sequences from both human and bovine G8 RVs (>82% nucleotide [nt] and >92% amino acid [aa] sequence identity). G8 isolates are commonly detected in cattle, unlike humans. This finding may be caused by the interspecies transmission of RVs between humans and cattle inducing natural reassortants. Sequence analysis of the VP4 genes of both strains revealed 89.6% nt and 97.1% aa sequence identity and greatest homology (>83% nt and >93% aa) to the published P[14] human RV strains. In addition, both strains exhibited 81%–86.9% nt and 91.8%–95.8% aa similarity to the lapine P[14] RV strains. Another report indicated that the G6P[14] genotypic combination was isolated for the first time from a 2-year-old Egyptian child [57], which was the first documented human G6 RV strain in Africa; this strain was designated EGY3399. Sequences of the NSP4 and NSP5 genes of this strain shared the highest similarity of bovine and simian origin, respectively. Moreover, the other genes encoding non-structural proteins were closely related to the genes of animal origin. Interestingly, the three aforementioned atypical Egyptian human P[14] RV isolates are closely related to the bovine G8 and G6 genotypes. A previous study concluded that depending on the VP7 gene sequence, there is a close association of group A RVs between bovine and humans in Egypt [103]. Sequencing of two VP7 isolates (one from a human and one from a calf) showed a high level of VP7 gene homology (95.3% nt and 97.6% aa sequence identity). Thus, this strong identity between the two strains showed that VP7s of bovine RV origin is shared in strains of human RVs. This finding confirms the interspecies transmission of bovine RV to humans, which may be due to the close contact between human and farm animals, especially in developing countries. Another unusual group A RV was detected and isolated for the first time in a stool sample from a child 6 months of age who had acute gastroenteritis in Egypt in 2012 [104]. Full genomic characterization by next-generation sequencing and phylogenetic analysis revealed that the AS997 strain had the consensus P[14] genotype constellation with G9, T1, and H1 reassortment. VP6 was most closely related to human and cat strains with a nt sequence identity of 96.0T and 94.5%, respectively, and clustered with other human strains. The AS997 strain VP2 genotype had a 95.3% nt sequence identity with antelope RVA and clustered with other human and bovine C2 strains.
Interspecies transmission of RV among animals was also confirmed in Egypt. A study reported RVA infection in dromedary camels in Egypt between 2004 and 2005 [86]. VP7 sequence analysis of the two isolates showed high shared identity to the G10 serotype of group A bovine RVs ranging from 90%–93%. More recently, a study investigated the RVA prevalence among diarrheic children and rats (15.4% and 3.3%, respectively) [105]. Notably, both human and rat sequences shared high identity (99% and 98%, respectively) with human RVA genotype G3P[8] considering that the G3P[8] genotype is the most prevalent human RVA genotype circulating among children in Cairo, Egypt [65].
CONCLUSIONS AND RECOMMENDATIONS
RV is the most common causative agent of acute diarrhea in children and animals worldwide. RV is also the main common cause of gastroenteritis in infants, young children, and calves in Egypt. There are currently various commercially-available diagnostic techniques for RV detection in clinical and environmental samples. ELISA and PCR are the most commonly used techniques for detecting, serotyping, and genotyping virus infections. As a result of low human vaccine coverage, the incidence of the RV infection is high in African countries. The same situation exists in animals because there are no commonly administered vaccines for animals in Africa. RV research and genotyping has not been given priority attention in Egypt. This review evaluated the epidemiology of G and P RV genotypes based on data between 1992 and 2022. All the available published studies on RV investigations in Egypt were retrieved from PubMed or other local databases. Variations in the distributions of the G and P genotypes were noted to be linked with temporal and geographic situations. Generally, we found limitations in the depth and quantity of investigations in Egypt. Bovine RV was shown to be a neglected disease in Egypt, although there is high calf mortality because of diarrhea in various regions. There are potent interactions between animal and human group A RV. The zoonotic surveys are limited due to rare availability of genome sequences of animal group A RV. The concurrent investigation of RVA infections in animals, including wild species, and humans, as well as the accumulation of nt sequences from animal strains are critical to recognize the biology, epidemiology, and evolution of such viruses.
Hence, based on this review, the subsequent points are recommended:
Continued human RV surveillance investigations should be performed to recognize new strains circulating in Egypt;
A human RV vaccine should be introduced into the National Immunization Program;
A human RV vaccine shall include the G9 strain as it is frequently detected;
A bovine RV in Egypt should be given more attention from the animal health policy for the implementation of investigations and research reports to enhance the productivity so that the country benefits from the animal health sector;
A bovine RV vaccine needs to be introduced into Egypt after detection and characterization of the different strains that cause calf diarrhea; and
Future studies, such as a 2-year long surveillance on both environmental and clinical samples with a phylogenetic analysis, are required to elucidate the epidemiology of RV.



