Introduction
Severe coronavirus disease 2019 (COVID-19) usually starts with flu like symptoms [1]; the disease can be asymptomatic, or may have a mild to severe course [2]. The infection is characterized by a substantial burden of inflammation [3]. An association between inflammation and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been confirmed in various reports [4, 5]. Because IL-6 and D-dimer are also associated with inflammatory conditions, they warrant study in the context of SARS CoV-2 infection.
Organ injury, including cardiac injury [2, 6–9], is common in patients with severe COVID-19 and is associated with poor survival probability [6, 10]. However, the underlying mechanisms remain unclear. Understanding of the mechanism that links COVID-19, which is caused by SARS-CoV-2 infection, with organ injury, might reveal upstream risk factors or biomarkers that may serve as potential therapeutic targets. Given the absence of an anti-viral treatment that directly targets the cardiac injury associated with the disease, an urgent need exists to develop such alternative therapeutic strategies.
SARS-CoV-2 is believed to induce the activation of immune and thrombotic pathways, which may contribute to organ injury [11–13]. A higher coagulation status or overactivation of immune pathways has been reported in patients with severe COVID-19 who died than survivors. In the former patients, levels of the inflammatory cytokine IL-6 and the thrombotic biomarker D-dimer are elevated early during hospitalization. Primary explorations of immune-related or coagulation dysfunction related cardiac injury in COVID-19 have been conducted. However, whether activation of immune or thrombotic pathways is preceded by cardiac injury has not been explored.
To investigate this possibility, we performed detailed longitudinal profiling of biomarkers of inflammation, coagulation, cardiac injury, and mortality in 170 cases of severe COVID-19 at Tongji Hospital in Wuhan, China. Early detection and monitoring of immune and thrombotic activation biomarkers may facilitate the prediction of cardiac injury in severely ill patients with COVID-19.
Materials and Methods
Patient Population
Patient selection, data extraction and validation, and the definition of cardiac injury have been detailed in our previous publication [14]. We previously reported findings in 1284 patients with COVID-19 pneumonia diagnosed with computed tomography who were transferred to Tongji Hospital between January 29, 2020, and March 8, 2020. At admission, circulating cardiac troponin I (cTnI) was measured in 1159 patients, and 170 (14.7%) patients with cardiac injury were reviewed in this study. Cardiac injury was defined according to the European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic [15], by blood levels of the cardiac biomarker high-sensitivity troponin I (hs-TnI) above the 99th-percentile upper reference limit, regardless of new abnormalities observed in electrocardiography and echocardiography. COVID-19 diagnosis was confirmed by laboratory testing performed according to the interim guidelines of the World Health Organization [16]. Blood specimens were collected within 72 hours after admission, depending on each patient’s clinical situation, for testing as required by the treating physicians.
Analysis of Inflammatory and Thrombotic Cascade Biomarkers
White blood cell (WBC), lymphocyte, and platelet counts (Sysmex XN9000, Japan); tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6; Cobas e602, Germany), C-reactive protein (CRP; Cobas 8000, Germany), D-dimer, and fibrinogen levels; and international normalized ratio (INR), activated partial thromboplastin time (APTT), and prothrombin time (Stago STA-R, France) were determined by the Tongji Hospital Clinical Biochemistry Laboratory.
Statistical Analysis
Elevated admission/peak cTnI levels are associated with poor COVID-19 prognosis [14]. In this study, we sought to explore the relationships among admission immune activation status, admission coagulation status, peak cTnI levels, and in-hospital outcomes. First, biomarker levels were compared according to the in-hospital outcomes (survivors vs non-survivors) among these 170 patients. Second, according to the dynamic changes in cTnI levels and survival bias, the patients were grouped into early mortality, late mortality, and survivor groups. Subsequently, immune activation and coagulation biomarker levels were compared between the survivor group and early-mortality group. Furthermore, the value of baseline biomarker levels in predicting in-hospital death and peak cTnI was explored.
Continuous variables are expressed as median (interquartile range [IQR]). The Mann-Whitney test was used for two-group comparisons, whereas Kruskal-Wallis test was used for comparisons among three groups. Categorical variables are expressed as proportions and percentages, and Fisher’s exact test was used to compare differences. The odds ratio (OR) and 95% confidence interval (CI) were separately calculated to predict in-hospital death with a multivariable regression model adjusted for age, sex, and comorbidities. A simple linear regression analysis was applied to examine the correlation between baseline biomarker levels and peak cTnI levels. Biomarker levels and dynamics over time were stratified by survival status, as follows: (1) survivors, (2) early mortality (≤21 days), or (3) late mortality (>21 days). Statistical significance was defined as P ≤ 0.05. Data analysis was performed in GraphPad Prism 7.00 (San Diego, CA, USA) software.
Results
Biomarker Characteristics of Study Patients at Admission
Table 1 demonstrates the biomarker characteristics at admission among study patients stratified into those discharged from the hospital (survivors, n = 49) and those who died in hospital (non-survivors, n = 121). Demographic information for patients who died or were discharged was recorded in our previous report [14]. Of the 121 patients with COVID-19 and cardiac injury who died, most died of progressive hypoxia due to pneumonia: 56 (46.3%) with respiratory failure, 23 (19.0%) with septic shock, and 6 (5.0%) with acute respiratory distress syndrome. Other causes of death were multiple organ dysfunction syndrome in 26 (21.5%), VT/VF in 6 (5.0%), and stroke or intracerebral hemorrhage in 4 (3.3%) patients. Levels of cTnI at admission did not differ between survivors and non-survivors (median [IQR], 39.4 (24.1, 69.43) pg/mL vs. 72.7 (19.85–351.6) pg/mL, P = 0.14), whereas N-terminal pro B-type natriuretic peptide (NT-proBNP) levels were elevated in non-survivors at admission (median [IQR], 1043 [457.5, 4550] pg/L vs. 485 [219, 1106] pg/L for survivors, P < 0.001). Coagulation abnormalities were observed in the non-survivor group after admission, in agreement with elevated D-dimer levels (median [IQR], 21 [11.16, 21] μg/mL vs. 2.47 [0.92, 12.65] μg/mL, P = 0.0005), increased PT (median [IQR], 15.3 (14.5, 17.7) s vs. 14.1 (13.55, 15.75) s, P < 0.001) and INR (median [IQR], 1.21 (1.11, 1.43) vs. 1.09 (1.03, 1.24), P < 0.001), APTT (median [IQR], 40 (36.3, 45.9) s vs. 37.7 (34.4, 42.4) s, P = 0.0139), and diminished platelet count (median [IQR], 167 (111, 231) × 109/L vs. 212 (143.8, 297.3) × 109/L, P = 0.0066). Fibrinogen levels did not significantly differ between groups (median [IQR], 4.58 (2.97, 6.27) g/L vs. 4.76 (3.95, 5.66) g/L, P = 0.77). Inflammatory cytokine and CRP levels at admission differed between the survivors and non-survivors. At admission, CRP (median [IQR], 100.8 [60.6, 169.3] mg/L vs. 51.1 [13.3, 77.6] mg/L, P < 0.0001), TNF-α (median [IQR], 10.7 [7.8, 17.3] mg/L vs. 8 [5, 11.8] mg/L, P = 0.02), and IL-6 (median [IQR], 68.01 [28.69, 192.6] vs. 17.27 [7.08, 41.1] pg/mL, P < 0.0001) levels were higher in non-survivors than in survivors (Table 1). Furthermore, in the early mortality cohort, men with cardiac injury had poorer prognosis than women (70.4% vs. 40%, P = 0.0014). In the early death cohort, cTnI levels (median [IQR], 591.9 [199.3, 1750] pg/mL vs. 201.6 [66.3, 1883] pg/mL, P = 0.0732) and NT-proBNP levels (median [IQR], 854 [413.8, 5015] pg/mL vs. 2070 [844.5, 5165] pg/mL, P = 0.2143) did not differ significantly between men and women, but men tended to have more severe cardiac injury. Admission IL-6 (median [IQR], 100.5 [34.1, 247.2] vs. 119 [46.33, 477] pg/mL, P = 0.6636), CRP (median [IQR], 118.1 [82.05, 167.2] vs. 93.2 [36, 163.8] pg/mL, P = 0.2088), and TNF-α (median [IQR], 12.15 [8.550, 17.70] vs. 13.55 [7.075, 25.73] pg/mL, P = 0.8289) levels did not significantly differ between men and women.
Demographics, Cardiac Injury, and Biomarker Characteristics of Patients with COVID-19.
| cTnI-positive survivors (n = 49) | cTnI-positive non-survivors (n = 121) | P value | |
|---|---|---|---|
| Age (years) | 61.5 (32, 69) | 64 (24, 70) | 0.27 |
| Male (%) | 16 (32.7%) | 77 (63.6%) | <0.001 |
| Onset of illness (days) | 11 (6.5, 11.5) | 11 (7, 15) | 0.58 |
| Cardiac injury and dysfunction biomarkers at admission | |||
| Baseline cTnI (pg/mL) (≤26.2 pg/mL) | 39.4 (24.1, 69.43) | 72.7 (19.85–351.6) | 0.14 |
| NT-proBNP (pg/mL) (<241 pg/mL) | 485 (219, 1106) | 1043 (457.5, 4550) | <0.001 |
| Thrombotic biomarkers at admission | |||
| D-dimer (μg/mL) (<0.5 μg/mL) | 2.47 (0.92, 12.65) | 21 (11.16, 21) | <0.001 |
| Platelets (×109/L) (125 × 109 to 350 × 109/L) | 212 (143.8, 297.3) | 167 (111, 231) | 0.0066 |
| Prothrombin time (s) (11.5–14.5)(s) | 14.1 (13.55, 15.75) | 15.3 (14.5, 17.7) | <0.001 |
| INR (0.8–1.2) | 1.09 (1.03, 1.24) | 1.21 (1.11, 1.43) | <0.001 |
| Fibrinogen (g/L) (2–4 g/L) | 4.76 (3.95, 5.66) | 4.58 (2.97, 6.27) | 0.77 |
| APTT (s) (29–42)(s) | 37.7 (34.4, 42.4) | 40 (36.3, 45.9) | 0.0139 |
| Inflammatory biomarkers at admission | |||
| IL-6 (pg/mL) (<7 pg/mL) | 17.27 (7.08, 41.1) | 68.01 (28.69, 192.6) | <0.0001 |
| CRP (mg/L) (<1, low risk of CVD; 1–3, moderate risk of CVD; >3, high risk of CVD; >10, suggestive of infection or inflammation) | 51.1 (13.3, 77.6) | 100.8 (60.6, 169.3) | <0.0001 |
| TNF-α (mg/L) (<8.1 mg/L) | 8 (5, 11.8) | 10.7 (7.8, 17.3) | 0.0025 |
Abbreviations: cTnI, cardiac troponin-I; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein; IL-6, interleukin-6; APTT, activated partial thromboplastin time; INR, international normalized ratio; NT-proBNP, N-terminal pro B-type natriuretic peptide; CVD, cardiovascular disease.
The normal reference intervals are in parentheses.
Bold values in the table indicate significant P values.
Analysis of Cardiac Injury Biomarkers
We tracked cTnI levels over 30 days and found them to be markedly elevated in patients who eventually died (Figure 1A). A sharp decrease at and after 21 day in the median cTnI levels in patients who died during hospitalization suggested a survival bias between patients who died before day 21 versus later. We stratified our analysis by comparing patients who died between day 6 after symptom onset, the earliest time correlating near to admission, and day 21 (early mortality cohort) with patients who died after day 21 (late mortality cohort). In the early mortality cohort, cTnI levels were high, and patients in the late mortality cohort had lower cTnI levels, similar to those of survivors (Figure 1B). A similar trend was observed in NT-proBNP levels, which were higher in patients in the early mortality group than in the survivor and late mortality groups (Figure 1C). These data suggested that an increasing trajectory of cTnI and NT-proBNP (beyond admission levels) predicted early mortality, and that patients who died after 21 days from illness onset had cTnI and NT-proBNP levels similar to those of survivors.
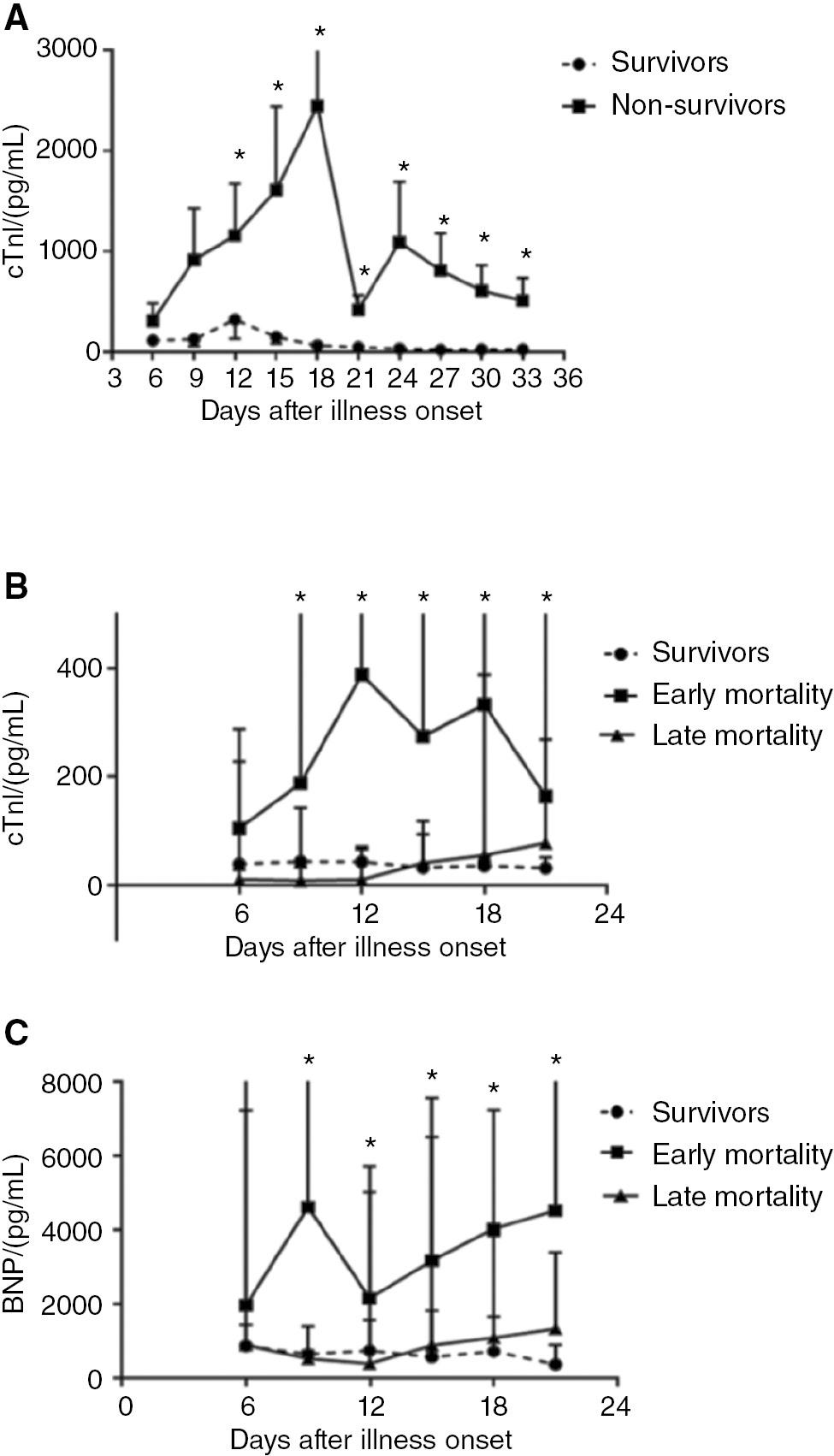
Cardiac Injury Biomarkers in Patients with COVID-19.
A. Temporal changes in cTnI levels among survivors and non-survivors with COVID-19.
B. Temporal changes in cTnI levels in survivors and patients with early and late mortality.
C. Temporal changes in NT-proBNP levels in survivors and patients with early and late mortality. *P < 0.05.
Evaluation of Immune Biomarkers
We focused on the early phases of the disease and observed that CRP levels markedly decreased over the period of hospitalization in survivors but remained elevated in patients with early mortality (Figure 2A). Elevated WBC and diminished lymphocyte counts correlated with early mortality (Figure 2B, C). Whereas TNF-α levels were elevated only at admission, IL-6 levels remained high in patients with early mortality (Figure 2D, E). In comparison with the late mortality group, the early mortality group had higher levels of IL-6 (median [IQR]: 23.86 [10.76, 85.78] vs. 179.4 [37.25, 736] vs. 15.23 [2.84, 41.6] pg/mL in survivors vs. early mortality vs. late mortality groups, respectively; P = 0.02), TNF-α (8 [7.3, 11.8] vs. 19 [12.45, 28.58] vs. 7.75 [4.78, 9.98] pg/mL in survivors vs. early mortality vs. late mortality groups, respectively; P = 0.003), and CRP (65.35 [23.6, 94.35] vs. 138.8 [111, 191] vs. 39.25 [36.61, 58.8] mg/L in survivors vs. early mortality vs. late mortality groups, respectively; P = 0.0004) (Figure 2F). These data suggested that early mortality in patients with COVID-19 was associated with the activation of the inflammatory immune cascade, a phenomenon already evident at admission. In addition, 12 (7%) patients had liver injury complications (elevated serum alanine aminotransferase [ALT] level), whereas 38 (22%) patients with kidney injury (increased serum creatinine level), and 7 (4%) patients had both liver and kidney injury.
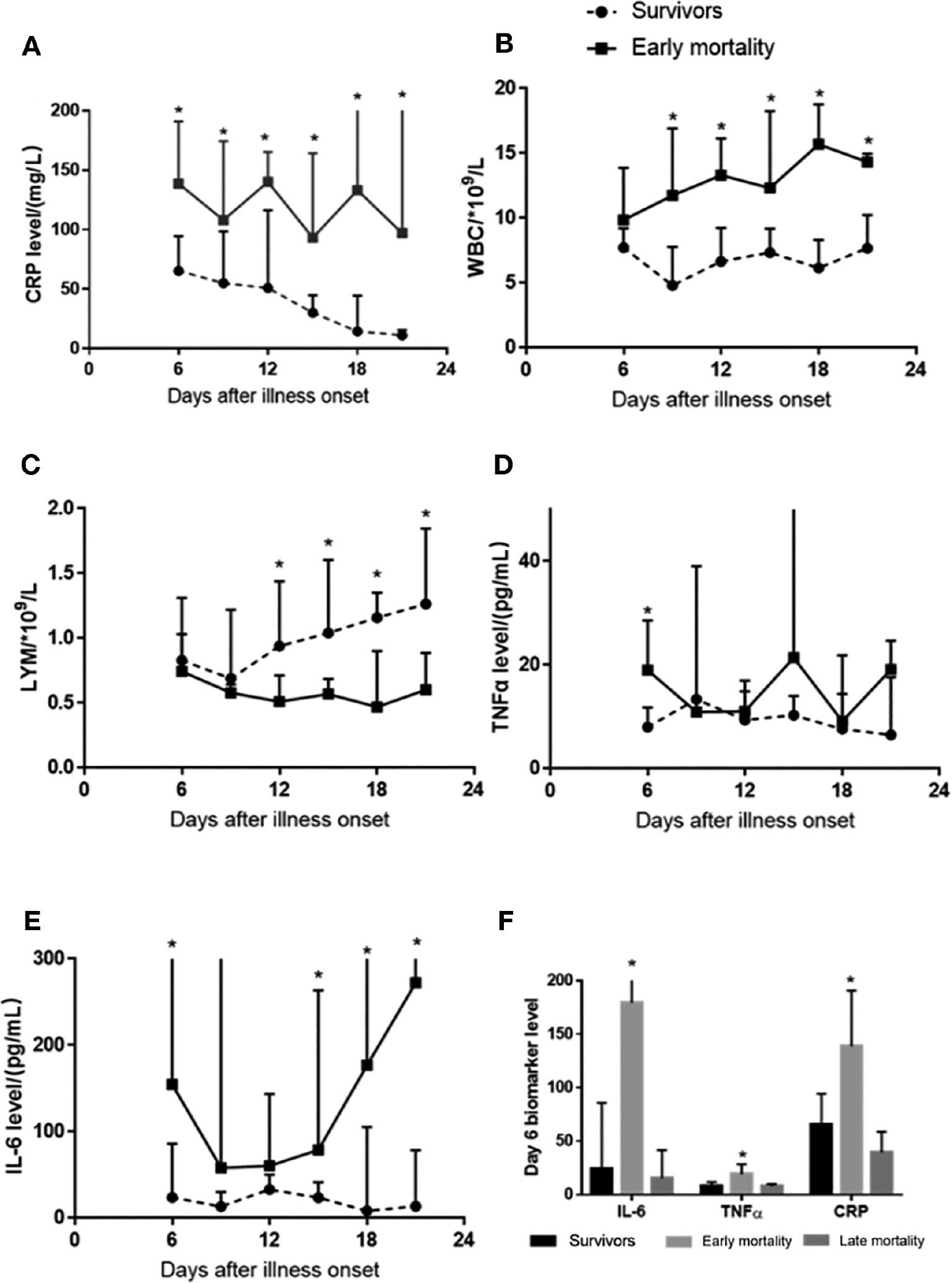
Biomarkers of Immune Activation in Patients with COVID-19.
A. Compared with survivors, patients with early mortality showed markedly higher C-reactive protein (CRP) levels during hospitalization.
B, C. Elevated WBC and decreased lymphocyte counts correlated with early mortality.
D. Tumor necrosis factor TNF-α levels were elevated only at admission.
E. Interleukin IL-6 levels remained elevated in patients with early mortality.
F. IL-6, TNF-α, and CRP levels at admission were compared between survivors and patients with early and late mortality. IL-6, TNF-α, and CRP levels were elevated only in patients who died early (F). *P < 0.05.
Evaluation of Thrombotic Biomarkers
The most notable abnormalities among thrombotic markers were the rapid increase in D-dimer levels from day 9 after illness onset, along with a rapid decrease in platelet counts beginning on day 12 among non-survivors (Figure 3A, B). APTT, prothrombin time, and INR increased over time in the early mortality cohort (Figure 3C–E), whereas no differences in fibrinogen levels were observed over the time course between non-survivors and survivors (Figure 3F).
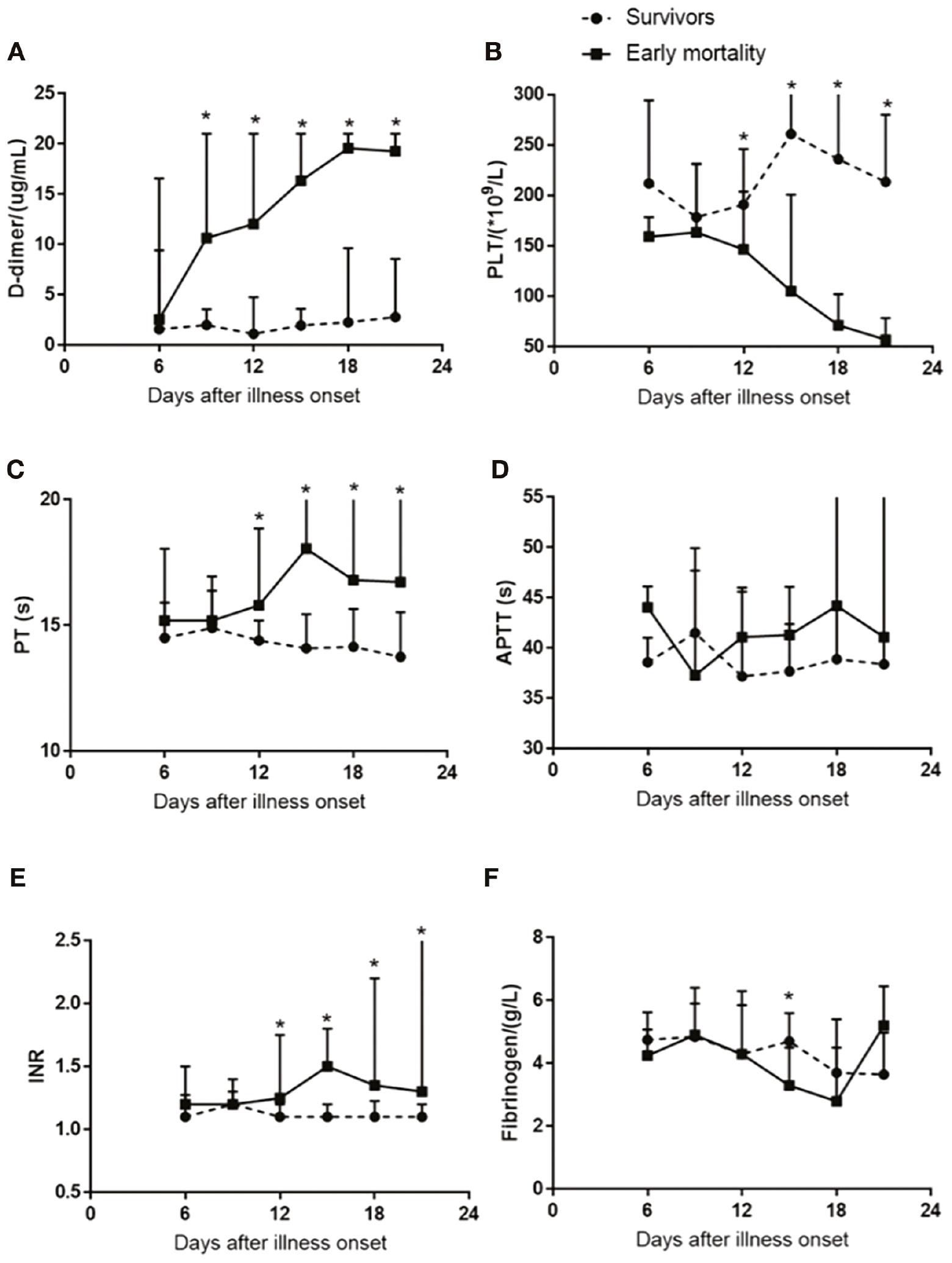
Activation Profile of Thrombosis Biomarkers in Patients with Cardiac Injury.
A, B. A prominent increase in D-dimer levels occurred from day 9 after illness onset, and platelet counts rapidly decreased on day 12 after illness onset in patients who died early compared with survivors.
C-E. International normalized ratio (INR), prothrombin time, and activated partial thromboplastin time (APTT) increased over time in the early mortality cohort.
F. No differences in fibrinogen levels were observed over time between groups (F). *P < 0.05.
Biomarkers Predictive of Cardiac Injury and Death
Table 2A describes the linear regression analysis results for the biomarkers of cardiac injury, according to peak cTnI values after adjustment for age; sex; and history of coronary heart disease, hypertension, and diabetes mellitus. Baseline IL-6 (β 0.243 [0.018, 0.468], P = 0.03) and D-dimer (β 0.27 [0.042, 0.50], P = 0.0021) levels predicted cardiac injury, thus suggesting of the ability of early immune and thrombotic activation to predict cardiac injury. We investigated the biomarkers that predicted mortality in a multi-variate model after adjusting for the aforementioned demographics/comorbidities, and found that the levels of D-dimer (OR 4.202 [2.15, 8.20], P < 0.001), IL-6 (OR 8.382 [3.28, 21.40], P < 0.001), and TNF-α (OR 9.012 [1.53, 52.94], P = 0.015), but not cTnI, at admission, predicted mortality (Table 2B).
Multivariable Regression Analysis of Death and Peak cTnI.
| A. Simple linear regression prediction of peak cTnI with D-dimer, IL-6, and TNF-α | |
|---|---|
| β* (95% CI*) | |
| log D-dimer | 0.271 (0.042, 0.500) |
| log IL-6 | 0.243 (0.018, 0.468) |
| log TNF-α | 0.125 (−0.498, 0.749) |
| B. Multivariate logistic regression analysis of death due to cardiac injury, thrombosis, and immunological parameters at admission | |
| OR* (95% CI*) | |
| log baseline cTnI | 1.35 (0.83,2.20) |
| log D-dimer | 4.202 (2.15, 8.20) |
| log IL-6 | 8.382 (3.28, 21.40) |
| log TNF-α | 9.012 (1.53, 52.94) |
*Adjusted by age, sex, coronary heart disease, and hypertension.
Abbreviations: TNF-α, (tumor necrosis factor-β); IL-6, (interleukin-6); cTnI, (cardiac troponin-I); CI, (confidence interval).
Discussion
Herein, we observed that cTnI levels continued to increase during the period of hospitalization, and peak cTnI values were several fold higher before death; these findings correlated with the significantly elevated D-dimer, IL-6 and TNF-α, and CRP levels observed early during hospitalization in patients with COVID-19 in the non-survivor cohort, thus suggesting that the activation of the immune and thrombotic pathways was preceded by cardiac injury. Our study provides a clear understanding of the pathogenic interactions among immune activation, coagulation, and cardiac injury in SARS-CoV-2 infection. In addition, or findings may help identify novel therapeutic targets to mitigate poor outcomes, and provide opportunities to improve clinical care and design therapeutic protocols for patients with severe SARS-CoV-2 infection and associated cardiac injury.
Emerging evidence from previous pilot studies suggests that lung injury is associated with elevated IL-6 in patients with COVID-19 with deteriorated lung function and an elevated need for mechanical ventilation [17–19]. Several cytokines and their receptors, such as TNF-α, IL-2, IL-2R, and IL-8, have been found to be elevated (single time point) in patients with more severe symptoms, and positively correlated with viral load and elevated cTnI levels [20, 21]. Moreover, in a large (1031 patients) retrospective study, He et al. [22] have found that patients with COVID-19 and associated acute myocardial injury have elevated IL-6 levels. Increased IL-6 levels and subsequent inflammatory cascade storms have been proposed, but remain to be validated, as the mechanism underlying cardiac injury and in-hospital death, particularly in patients with severe COVID-19. Our results demonstrated that IL-6 and TNF-α are key biomarkers reflecting the cardiac status of patients with COVID-19 early during hospitalization; these markers may identify patients with cardiac injury risk before the rise in cTnI level. Although immune activation is suspected to be another mechanism that accompanies respiratory complications in COVID-19, its exact association with cardiac injury is unclear. Emerging evidence has implicated dysregulation, and not simply overactivation, of the immune response. For instance, a detailed immunophenotyping study in 56 patients with COVID-19 has indicated that those with severe respiratory failure exhibited a marked decrease in human leukocyte antigen (HLA-DR) levels, which appears to be driven by sustained IL-6 and TNF-α production [23]. However, this pattern is not observed in severe bacterial pneumonia. Moreover, while inflammatory cytokines (IL-6, TNF-α, etc) dominate, the relative absence of a type I interferon response needed for viral clearance [17, 24, 25] suggests a pattern specific for SARS-CoV-2.
Whether alone or in combination, various inflammatory factors can induce cardiac injury. The SARS-CoV-2 specific inflammatory milieu may lead to cardiac dysfunction. Viral myocarditis or the recently described viral infection of endothelial cells [26] potentially leading to microvascular injury is another plausible mechanism. Several endothelial protective drugs have been shown to ameliorate clinical manifestations in patients with COVID-19 [27]. Whereas IL-6 levels were elevated in patients with a risk of early mortality, the extremely high IL-6 levels observed in our study might be directly associated with uncontrolled viremia, as observed elsewhere [28]. Receptor antagonists of these cytokines may attenuate inflammatory action and prevent escalation of cytokine storms, thereby suppressing the extent of cardiac injury in SARS-CoV-2 before the development of uncontrolled viremia. High IL-6 is also a known biomarker of systemic immune activation [29], which might damage multiple organs. However, our results indicated a more specific IL-6-associated cardiac injury with a relatively low incidence of complications of other organ injuries (7% complicated with liver injury; 22% complicated with kidney injury). This possibility should be further explored, given the limitation of the bias due to inclusion of only patients with cardiac injury in this study.
Coagulation disorders in patients with COVID-19 are strongly associated with poor prognosis, and various lines of evidence suggest that this association might be due to the prothrombotic state. Elevated D-dimer may be a biomarker of this pathway [6], although D-dimer elevation in COVID-19 may be multi-factorial [30]. In a case series of 183 patients with COVID-19, non-survivors (11%) exhibited elevated D-dimer and fibrin degradation products; 15 non-survivors met the criteria for disseminated intravascular coagulation, whereas only one survivor developed disseminated intravascular coagulation [31]. Similar derangements have been documented in a separate case series of 94 patients with COVID-19 [32]. Acute pulmonary embolism and deep venous thrombosis have been reported in patients with SARS-CoV-2 infection [33, 34]. The limited autopsy data suggest a constellation of pathological findings, including thrombus formation in pulmonary microvessels [35]. Therefore, D-dimer levels were higher and tended to rise to a greater extent among non-survivors. Consequently, patients with COVID-19 may exhibit an underlying pro-thrombotic state. In a small case series of 15 individuals, Bois et al. [36] have observed that post-mortem fibrin microthrombi were more common (80%) than acute ischemic injury (13%) and myocarditis (33%), thus suggesting a role of thrombosis in accentuating myocardial injury. Previous studies have reported a progressive rise in D-dimer and cTnI levels in patients who subsequently died but not in survivors [6]. Patients with D-dimer values higher than 1 mcg/mL at admission had elevated risk of in-hospital death (adjusted OR: 18.42; 95% CI: 2.64, 128.55; P = 0.0033). A comparison between 113 COVID-19 non-survivors and 161 survivors by Chen and colleagues has indicated markedly higher cTnI and D-dimer levels in non-survivors [10]. In several other retrospective/observational studies, elevated D-dimer levels and their relationship with cardiac injury have also been confirmed [37–40]. Furthermore, the peak cTnI level during hospitalization is positively associated with D-dimer level [38]. Moreover, in a short-term follow-up study, low D-dimer levels at cardiac injury onset have been found to predict post-discharge recovery [40]. With a deepened understanding of the comprehensive hyper-coagulation status in patients with COVID-19, emerging evidence supports anti-coagulation therapy [41–43]. Prophylactic use of parenteral anticoagulants during hospitalization is recommended [44], and a consensus is emerging regarding the role of in-hospital heparin as primary thromboprophylaxis [45]. Specifically, the relative benefits of different dosing regimens remain unclear in the management of patients with COVID-19 with associated cardiac injury.
Limited retrospective studies have described the interplay between cardiac injury and immune and thrombotic disorders [6, 22, 46]. In a large population-based study by Li et al. [46], higher cTnI, IL-6, and D-dimer levels have been observed in critically ill patients and deceased patients. Additionally, biomarker phenotyping analysis has indicated that hypersensitive TnI is positively associated with IL-6 and D-dimer levels. This study further implied that myocardial injury may be mediated by cytokine release syndrome. The accompanying rise in cTnI, IL-6, and D-dimer levels has also been verified by He et al. [22]. Both studies have indicated a direct relationship between immune and thrombotic dysfunction and cardiac injury in COVID-19. Hyper-coagulation status and cardiac injury have also been found in patients with severe Delta variant COVID-19, beyond the primary strain [47]. Furthermore, elevated cTnI and D-dimer levels in patients with Delta variant infection have been associated with adverse outcomes [47, 48]. From our study, we infer that cTnI levels detected in SARS-CoV-2 infection are associated with elevated immunological and coagulation biomarkers, and poor prognosis. Such risk stratification is of utmost importance in avoiding poor prognosis. Biomarkers such as cTnI, IL-6, and D-dimer may be valuable in assessing patient prognosis, because of their ability to predict the clinical course and outcome. Further studies remain necessary to clearly define the pathogenesis of COVID-19, and its different strains and variants, to advance understanding of the mechanism of cardiac injury in patients with severe COVID-19; enable early identification of high-risk patients for appropriate resource allocation; and provide tailored therapies that result in better prognosis among patients affected with SARS-CoV-2 and its strains in the future.
Sex-related differences have also been compared. To date, no consensus has been reached regarding sex-associated outcome differences in patients with severe COVID-19. In the 2003 Severe Acute Respiratory Syndrome (SARS, caused by SARS-CoV-1 infection) epidemic, sex differences have been observed, on the basis of a lower risk of death in women than men [49]. Analogous observations have been made for SARS-CoV-2 infection, for which, men have a higher mortality risk than women [50, 51]. However, the COVID-19 case fatality rate is higher in women than men in several countries, such as India [52]. In our study, men with cardiac injury had poorer prognosis than women, but the inflammatory states were not significantly different.
Finally, this research has several limitations. To prevent the spread of COVID-19, the assessment of ventricular function by echocardiography to determine cardiomyopathy was not routinely performed. Therefore, we were unable to correlate the rising cTnI levels with cardiac contractile function. We reviewed the data for only cTnI-positive patients. The collection of biomarker testing was not periordically as required for the research and was left to the discretion of the COVID-19 healthcare team, thus potentially introducing a bias. For instance, of the 170 patients with elevated cTnI levels, 38 had no baseline IL-6 or D-dimer level data, because of the lack of experience of the pandemic medical responders.
Conclusions
Cardiac injury in patients with COVID-19 is closely associated with the activation of immunological and thrombotic pathways, and can be predicted by biomarkers of these pathways at admission. This study supports a strategy of biomarker-guided, point-of-care therapy. Further randomized studies are warranted to develop anti-immune and antithrombotic treatment regimens for patients with severe COVID-19 and cardiac injury.

