Pulsed field ablation (PFA) is an exciting new technology with substantial potential to change ablation of arrhythmia, particularly atrial fibrillation (AF) [1–3]. It exploits the localized application of intermittent, high-intensity electric fields for short periods of time (micro- or nanoseconds), thus resulting in cellular and tissue electroporation. According to the theory of electroporation, the electric field induces a voltage across the cell membrane bilayer and decreases the energy required for spontaneous formation of aqueous pores in the bilayer, thus facilitating the formation of more pores. Pore formation finally leads to permeabilization, which can be reversible or irreversible, depending on the parameters of the applied PFA [4]. At higher energy levels, it can cause devastating leakage of the intracellular contents, such that cell viability is lost. PFA uses this phenomenon to ablate the myocardium (Figures 1 and 2).
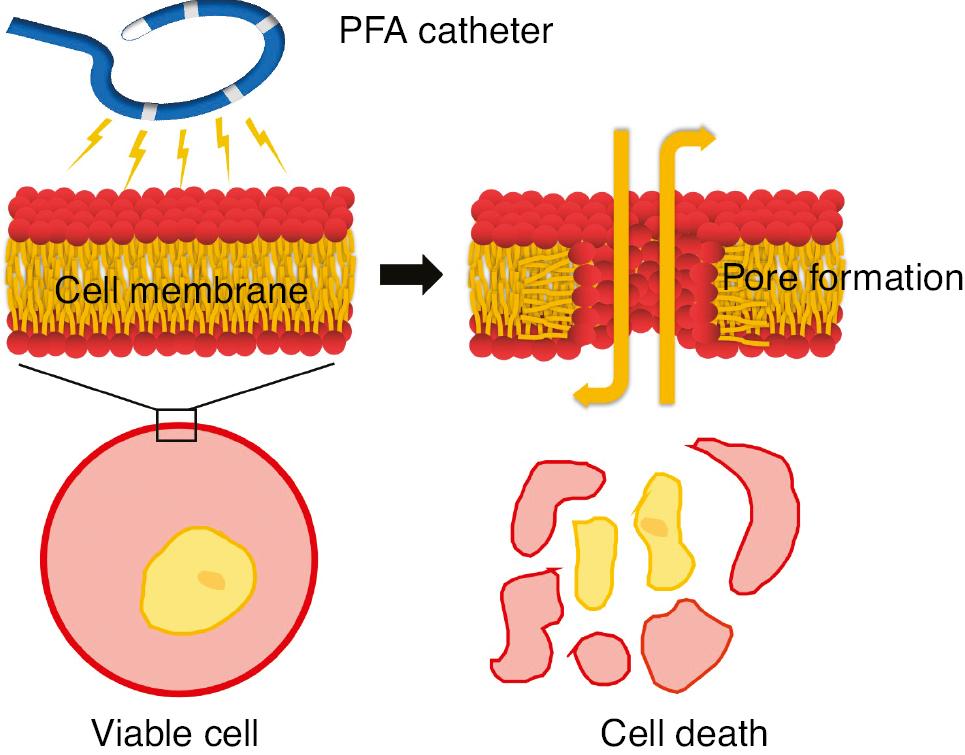
Molecular Simulation of Pore Formation with an Electric Field Produced by a Pulsed Field Ablation (PFA) Catheter.
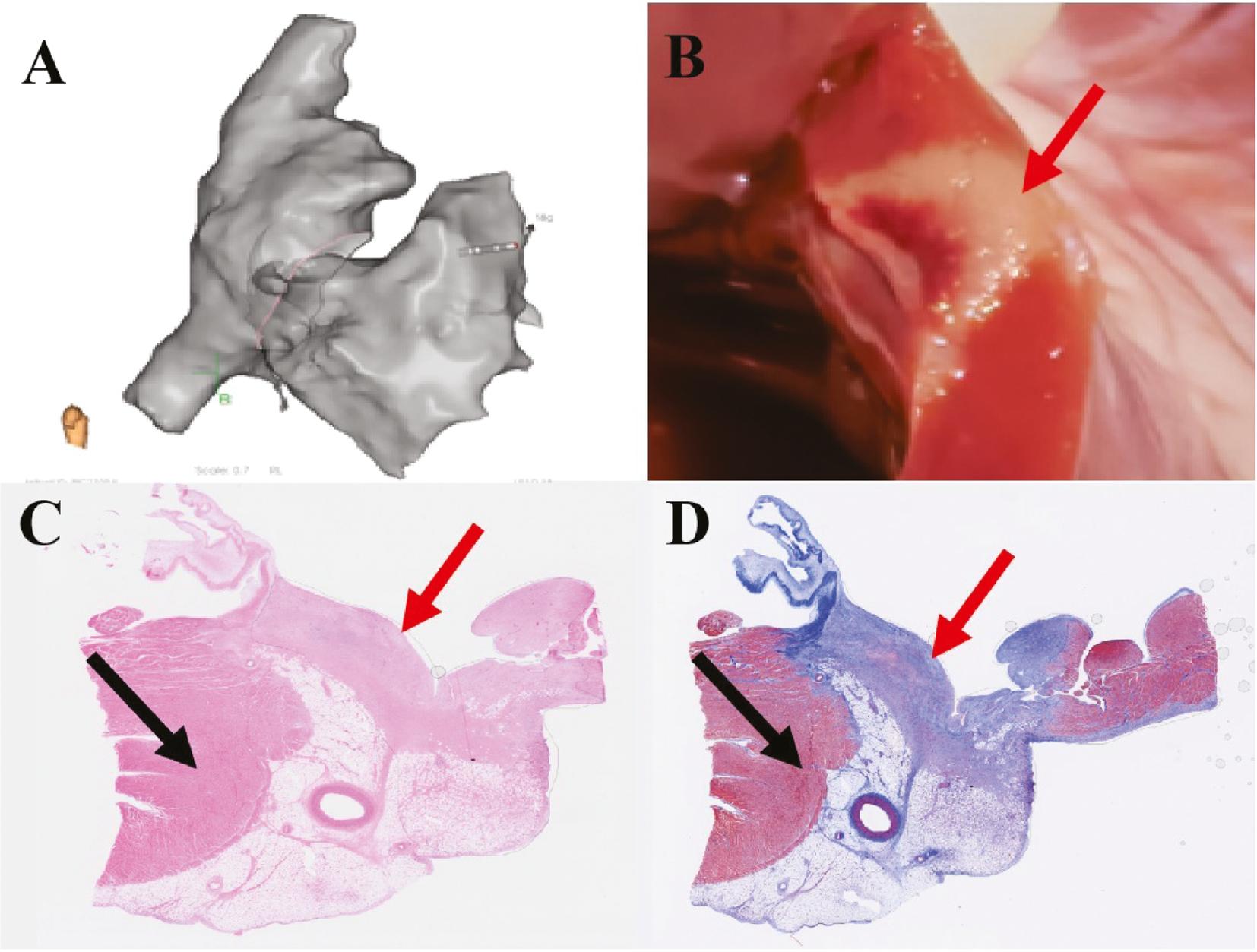
PFA Creates Homogeneous Transmural Lesions in Ventricular Myocardium.
(A) Three-dimensional model of the right heart during PFA. (B) Gross appearance of the endocardial surfaces of PFA lesions. (C) PFA lesions after hematoxylin eosin staining. (D) PFA lesions after Masson’s trichrome staining. The red arrow indicates PFA lesions, and the black arrow indicates normal myocardium.
The most promising feature of PFA is its tissue specificity. The electric field strength threshold required for cell death varies among tissue types and is relatively very low for myocardium. Thus, by using the ablation energy in the range for myocardium, PFA therapy can be conducted without causing injury to collateral structures. PFA is widely considered a non-thermal means of ablation, but it is not inherently nonthermal. It can generate heating in tissue through Joule heating from the delivery of electric fields and the resulting current flow through a resistive tissue load. PFA therapy relies on delivering the electrical energy in a manner sufficient to induce cell death to the targeted region without exposing the tissue to excessive heating, thereby minimizing the risk of injury to surrounding structures. Another advantage is that, in comparison to radiofrequency ablation, PFA does not rely on tissue-catheter contact for efficacy; however, poor tissue proximity may generate more injury for a given set of PFA parameters.
In recent years, several clinical studies with strict study protocols have been published on PFA in patients with paroxysmal AF. PFA systems such as Farawave, Sphere-9 and PulseSelect have been demonstrated to be both feasible and safe in AF ablation [5–7].
Those ablation systems primarily use basket or flower-shaped catheters, which are not integrated in a three-dimensional system and require placement under fluoroscopic guidance, as well as the use of a bulky steerable sheath for manipulating the ablation catheters.
Few data are available regarding the clinical applications of annular-shaped PFA catheters. In July 2021, we performed a clinical trial (Clinical trials: NCT05400928) on the use of an annular-shaped PFA catheter and a three-dimensional system integrating mapping and ablation, called LEAD-PFA (Sichuan Jinjiang Electronic Medical Device Technology Co., Ltd. Chengdu, China), for paroxysmal AF ablation. Within the first 2 months, more than 50 cases of paroxysmal AF were treated with PFA therapy via this system, and more than 150 PFA procedures were subsequently completed as part of this trial.
Most domestically designed PFA systems use circular catheters, integrating mapping and ablation function. The main advantages of this method are as follows: (1) three dimensional mapping and ablation are integrated and can be performed directly in one map; (2) one catheter can be used for both mapping and ablation, thus decreasing catheter exchanges; (3) the annular-shaped catheter may slightly decrease ablation efficiency and increase the number of ablations. However, owing to the shorter catheter exchange time and the identification of catheter location through X-ray positioning, the overall efficiency of PFA system is substantially improved; (4) the design of the circular catheter allows for direct application of an 8.5 F atrial septal puncture sheath, which may decrease the catheter exchange time. In addition, it may allow for better handling than a 12/13 F catheter with basket or flower-shaped electrodes, thereby decreasing the potential risk of tears caused by bulky catheters.
During the PFA procedure in our center, although the first four cases received general anesthesia, the subsequent 50 cases were performed under local anesthesia. A biphasic waveform was used for PFA pulse delivery and was found to greatly decrease muscle contraction. Most patients showed no substantial discomfort during the procedure, and only 10–20% of patients had clear vasovagal reactions during the ablation of the anterior wall of the left superior pulmonary vein, manifesting as a decrease in heart rate and a mild decrease in blood pressure. Generally, after intravenous injection of 1 mg atropine, and appropriate acceleration of the speed of fluid infusion, the vasovagal reactions in those patients were effectively relieved (Figure 3).
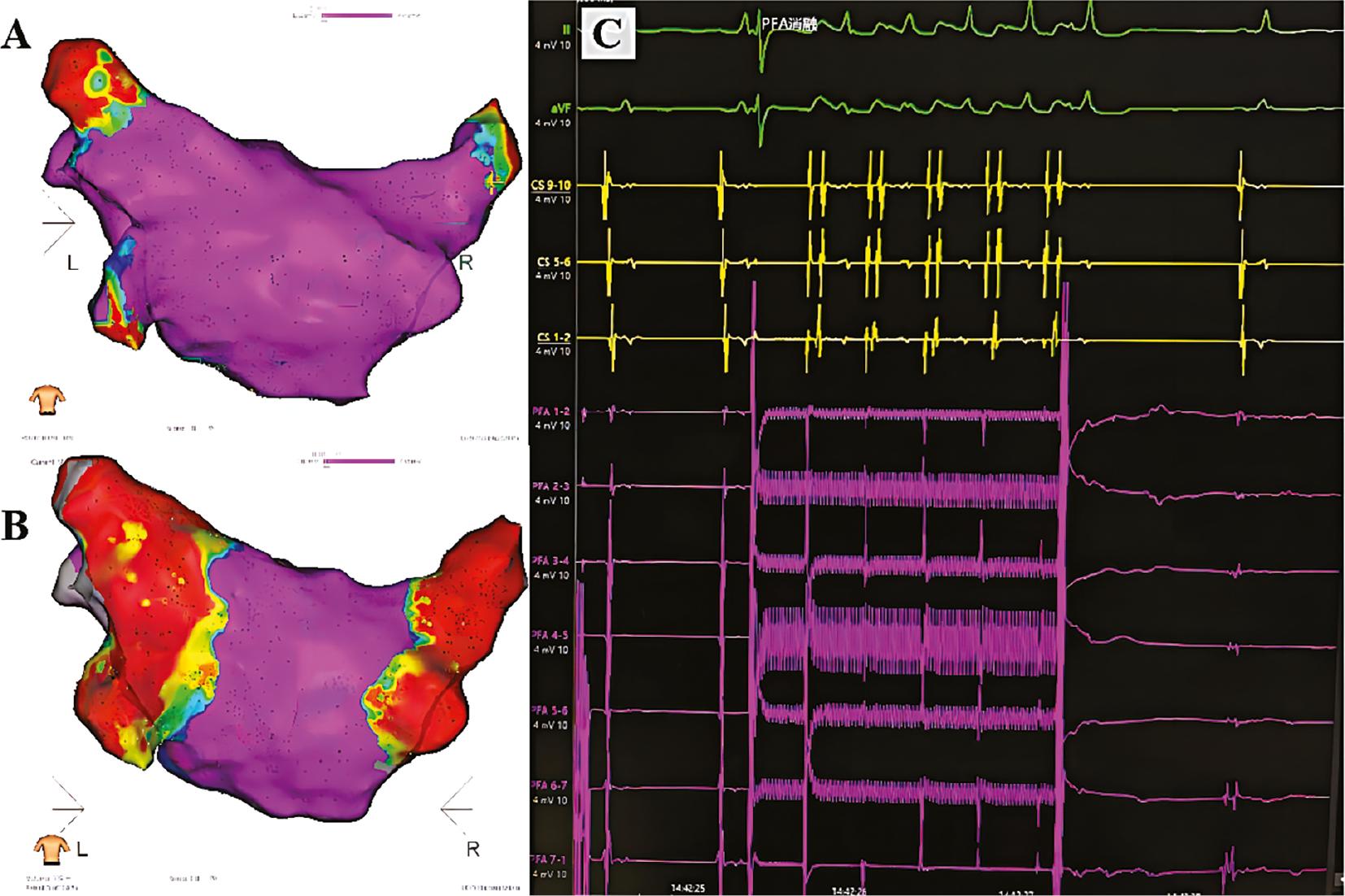
Isolation of Pulmonary Veins with PFA.
Electroanatomical voltage maps of left atrial and pulmonary venous model before (A) an after PFA (B). Pulmonary vein potentials recorded from bipolar electrograms from the electrode array are shown immediately before and after one pulsed field application (C).
In terms of safety, we assessed the risk of microbubble generation by PFA. Under esophageal Doppler monitoring, we observed no clear bubbles during the PFA procedure, even at the highest energy setting in this ablation system. Although small amounts of microbubbles were observed in some patients, no patients experienced a stroke, and their head MRI findings indicated no cause for concern. In addition, no other serious complications were observed. In terms of effectiveness, all patients enrolled in our center completed the 1-year follow-up, and the success rate exceeded 95% after initial statistical analysis.
Although this PFA system performed well during AF ablation, some problems still require further exploration. First, although PFA does not strictly require tissue-catheter contact, on the basis of previous research results, improper contact might decrease the efficiency of tissue damage. Second, the circular-shaped catheter made maintenance of catheter stability challenging during ablation at the top of the right superior pulmonary vein. In addition, the current catheter design might not be ideal for ablation of the isthmus of tricuspid valve when a patient has both AF and atrial flutter.
Further modifications including the application of tissue-catheter contact parameters, and the combination of a steerable sheath should result in substantially better effects. Finally, we believe that a three-dimensional system integrating mapping and ablation will provide a new paradigm for electrophysiologists.

