Introduction
Despite widespread awareness and use of scientifically proven life-prolonging medical and device-based therapies over the last two decades, heart failure remains a leading cause of morbidity, mortality, and health care expenditure in the United States [1]. Patients who have progressive heart failure syndromes despite receiving standard treatment, including medical therapy and cardiac resynchronization, are being increasingly considered for implantation of continuous-flow left ventricular (LV) assist devices (CF-LVADs). CF-LVADs are effective in improving survival and quality of life in patients with advanced heart failure, and can be used as destination therapy or as a bridge to heart transplantation [2, 3]. The increasing incidence of CF-LVAD implantation over the last decade mandates an effort from all practicing physicians, not just heart failure specialists, to understand the basic anatomic and physiologic implications of these devices on cardiac structure and function. Echocardiography plays an integral role in the evaluation of patients with advanced heart failure who are being considered for or are mechanically supported by a CF-LVAD. Here we provide a focused clinical review on the use of echocardiography in two main aspects of the evaluation of these patients: (a) optimal patient selection for CF-LVAD support and (b) follow-up assessment of optimal pump function.
Optimal Patient Selection
In patients being considered for durable CF-LVAD implantation, the preoperative echocardiogram is a critical part of the evaluation and can be a powerful predictor of short-term and long-term clinical outcomes after implantation. The preoperative echocardiogram should focus on the following:
Left Ventricular Structure and Function
Patients being considered for CF-LVAD placement almost invariably have severe reduction in their LV systolic function. Consideration of mechanical circulatory support, including CF-LVADs, is not recommended if the LV ejection fraction is greater than 25% [4]. Careful measurement of LV dimensions is also important, as the presence of relatively small LV cavities, specifically smaller than 63 mm, has been independently associated with increased morbidity and mortality after CF-LVAD implantation [5]. This is likely related to excessive LV emptying and exaggerated leftward shift of the interventricular septum due to its close proximity to the inflow cannula. This abnormal septal deformation worsens RV function [6], and can also cause direct physical contact of the septum with the inflow cannula, termed a suction event. Suction events prevent optimal function of the CF-LVAD as they automatically trigger speed decrements and are frequently associated with ventricular arrhythmias [7]. From a structural and functional standpoint, hearts with a severely reduced LV ejection fraction and moderate to severe degrees of LV dilation are therefore likeliest to benefit from CF-LVAD support.
Right Ventricular Structure and Function
Assessment of RV function is critical in patients being considered for LV assist device (LVAD) implantation. The hemodynamic effects of a CF-LVAD on the right ventricle can be divergent. The desired favorable effect is improvement in RV performance following unloading of the left side of the heart and subsequent decongestion of the pulmonary circulation. However, several hemodynamic consequences on the right ventricle following initiation of CF-LVAD support challenge the right side of the heart. Three potentially detrimental effects on right-sided heart function are (1) acute increase in right ventricular preload (which requires an equivalent increase in right-sided cardiac output), (2) leftward septal deformation resulting from LV unloading leading to worsening RV function, and (3) worsening tricuspid regurgitation.
There are numerous validated echocardiographic measures of RV structure and function [8]. This review will focus on two indices that have been studied in CF-LVAD patients, are reproducible, and are easily obtainable: (a) tricuspid annular plane systolic excursion (TAPSE) and (b) heart rate–corrected duration of tricuspid regurgitation (TRDc).
TAPSE represents the distance of longitudinal motion of the tricuspid annulus during systole and is a validated index of RV systolic function [8]. It is obtained by M-mode imaging of the lateral tricuspid annulus obtained from an apical four-chamber view. An example is illustrated in Figure 1. TAPSE values below 7.5 mm have high specificity for postoperative right-sided heart failure, and may indicate a need for more aggressive preoperative hemodynamic optimization and/or the need for prolonged inotropic support following CF-LVAD implantation [9].
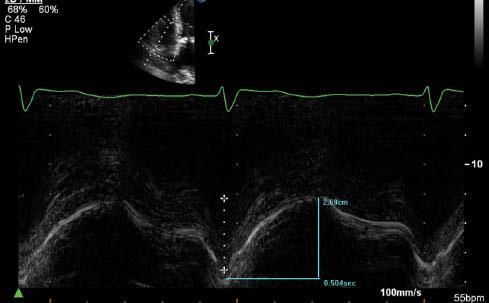
Assessment of Right Ventricular Systolic Function by Tricuspid Annular Plane Systolic Excursion (TAPSE) Measurement. The Longitudinal Motion of the Lateral Tricuspid Annulus is 27 mm, Consistent with Normal Right Ventricular Systolic Function (Normal is More Than 16 mm).
TRDc is a novel index described by Topilsky et al. [5] in a large cohort of CF-LVAD patients from the Mayo Clinic. One calculates it by measuring the duration of the tricuspid regurgitant Doppler jet and dividing it by the square root of the R-R interval. TRDc is a heart rate–adjusted measure of the time to pressure equalization between the right ventricle and the right atrium during systole that integrates right atrial compliance and the severity of tricuspid regurgitation. In this regard, it can predict the hemodynamic impact of an acute volume load increase on the right atrium, an expected result of CF-LVAD implantation. Not surprisingly, it was found on multivariate analysis to have a strong ability to predict rates of RV failure and death following LVAD implantation. Patients with a TRDc of less than 461 ms had an adjusted 2-year mortality odds ratio of 2.3 compared with patients with a TRDc longer than 461 ms. Examples of TRDc that would predict low and high risks of RV failure and death are illustrated in Figure 2.
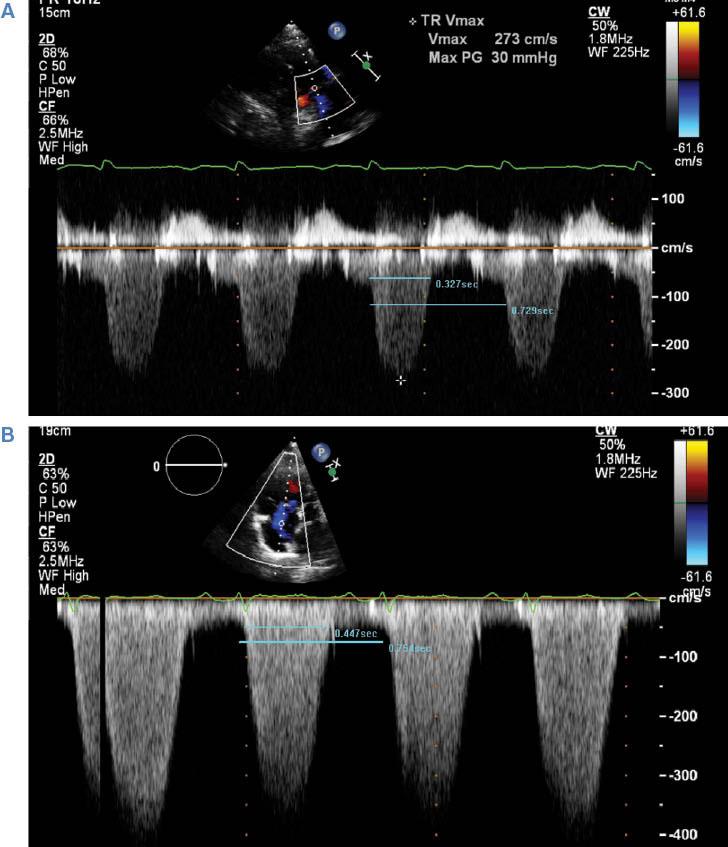
(A) Example of a Patient with a Heart Rate–corrected Tricuspid Regurgitation Duration (TRDc) Conferring a High Risk of Postoperative Right-sided Heart Failure and Death (TRDc = 384 ms). (B) Example of a patient with a TRDc conferring a low risk of right ventricular failure and death (TRDc = 519 ms).
Valvular Insufficiency
Accurate assessment of any underlying valvular regurgitation, particularly of the aortic and tricuspid valves, is crucial in the evaluation of patients being considered for CF-LVADs, as it directly impacts decisions to perform additional surgery at the time of implantation.
Aortic Regurgitation
There are several validated quantitative and qualitative methods for assessing the severity of aortic insufficiency (AI) [10]. Quantitative methods include regurgitant orifice and regurgitant volume calculation, which can be done with spectral Doppler imaging or the proximal isovelocity surface area method, and vena contracta or jet width measurement. Qualitative measures include pressure half-time and degree of descending aorta diastolic flow reversal. A brief summary of quantitative and qualitative estimates of AI severity is given in Table 1. It is important to remember that in patients with advanced heart failure, LV filling pressures are almost invariably elevated and the pressure half-time method may overestimate the severity of AI significantly [11]. The severity of any preexisting AI will typically worsen to some degree following initiation of CF-LVAD support [12]. Although CF-LVAD support is in many cases well tolerated in patients with minimal or mild AI, the presence of moderate AI or worse can result in a physiologically ineffective circuit (left ventricle → inflow cannula → outflow cannula → ascending aorta → left ventricle) owing to a large fraction of the blood volume exiting the outflow graft regurgitating into the left ventricle [13]. For this reason, any patient with AI of more than mild severity should also undergo concomitant aortic valve closure at the time of LVAD implantation. Repair with a single coaptation stitch at the time of implantation provides effective and durable repair of moderate or severe AI in patients in whom a CF-LVAD has been implanted [14].
Echocardiographic Parameters Used in Grading the Severity of Aortic Regurgitation.
| Parameter | Mild | Moderate | Severe |
|---|---|---|---|
| Vena contracta (cm) | <0.3 cm | 0.3–0.60 | >0.6 |
| Jet width (% of LVOT width) | <25 | 25–64 | >65 |
| Regurgitant volume (ml/beat) | <30 | 30–59 | >60 |
| EROA (cm2) | <0.1 | 0.1–0.29 | >0.30 |
| Descending aorta diastolic flow reversal | Brief, early diastolic | Intermediate | Holodiastolic |
| Pressure half-time (ms) | >500 | 200–500 | <200 |
EROA, effective regurgitant orifice area; LVOT, left ventricular outflow tract.
Tricuspid Regurgitation
A detailed assessment of tricuspid valve structure and function is key in the preoperative assessment of patients being considered for CF-LVAD implantation, particularly when there is any significant degree of tricuspid regurgitation. Similarly to its effects on overall RV function, the hemodynamic effects of CF-LVAD support on tricuspid regurgitation may result in worsening, unchanged, or improved tricuspid regurgitation severity [15, 16]. The acute increase in RV end-diastolic volumes following CF-LVAD support can cause functional tricuspid regurgitation to worsen, leading to ineffective forward flow and a syndrome of progressive RV failure. The severity of tricuspid regurgitation at the baseline is associated with increased risk of RV failure when it is moderate or worse on the preoperative transthoracic echocardiogram [17]. However, there are conflicting data regarding the benefits of tricuspid valve repair at the time of CF-LVAD implantation in patients with moderate or severe tricuspid regurgitation, and the decision to perform concomitant tricuspid valve repair is ultimately deferred to the performing surgeon [18–20]. Tricuspid regurgitation severity can be graded quantitatively by vena contracta width or tricuspid regurgitant jet area, or qualitatively by evaluation of the Doppler profile of the hepatic vein (systolic flow reversal implies severe tricuspid regurgitation) [10]. An illustration of severe tricuspid regurgitation by color Doppler imaging as well as hepatic vein systolic flow reversal is illustrated in Figure 3. Table 2 briefly summarizes the parameters of tricuspid regurgitation severity.
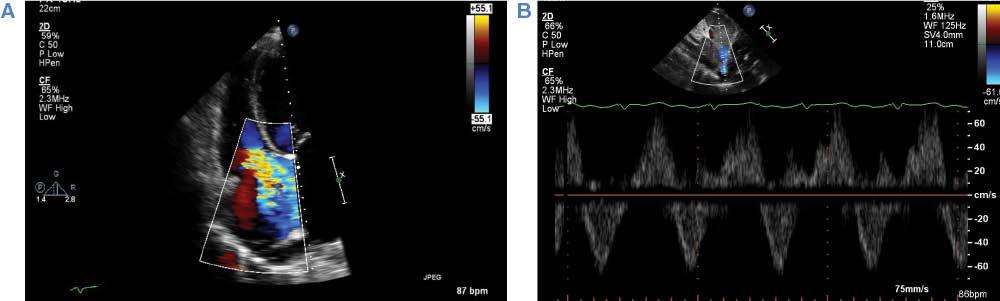
(A) Example of severe tricuspid regurgitation by color Doppler imaging. Note the broad-based regurgitant jet reaching the posterior wall of the right atrium. (B) Pulsed-wave Doppler imaging demonstrating hepatic vein systolic flow reversal, consistent with severe tricuspid regurgitation.
Echocardiographic Parameters Used in Grading the Severity of Tricuspid Regurgitation.
| Parameter | Mild | Moderate | Severe |
|---|---|---|---|
| Vena contracta (cm) | Not defined | Not defined, but <0.7 | >0.7 |
| Jet area (cm) | <5 | 5–10 | >10 |
| Doppler profile | Soft, parabolic | Dense, variable contour | Dense, early peaking, triangular |
| Hepatic vein flow Doppler | Systolic dominant | Systolic blunting | Systolic flow reversal |
Evaluation for Intracardiac Thrombi
Preoperative identification of intracardiac thrombus, particularly in the LV apex, which is not uncommon with dilated cardiomyopathy and a severely reduced LV ejection fraction, is also an important part of preoperative planning before CF-LVAD implantation. Although the presence of an apical thrombus does not contraindicate placement of a CF-LVAD, it requires removal of the thrombus at the time of surgery before insertion of the inflow cannula in the LV apex. One study reported that of 100 patients in whom an LVAD has been implanted over 3 years, six had an LV apical thrombus identified preoperatively or intraoperatively. None of them experienced a neurological event, pump thrombosis, or pump malfunction [21]. LV thrombi can be readily detected by transthoracic echocardiography, typically in the apical views. If images are of limited quality, echocardiography contrast agents should be used as they significantly improve the sensitivity, specificity, and accuracy of echocardiography in diagnosing LV thrombus [22]. An example is shown in Figure 4.
Presence of Intracardiac Shunts and Patent Foramen Ovale
The echocardiogram before CF-LVAD implantation should include careful inspection for any evidence of intracardiac shunting, including a patent foramen ovale (PFO). A PFO are not uncommon and has a reported prevalence of up to 25% in the general population [23]. Although it is typically noted incidentally and not felt to cause any significant shunting, the acute lowering of left-sided intracardiac pressures resulting from CF-LVAD support can precipitate increased right to left interatrial shunting and clinically important hypoxemia and cyanosis [24]. Preoperatively, transthoracic echocardiography can identify atrial level communication with color Doppler imaging of the interatrial septum, or in the apical windows following intravenous administration of agitated saline [25]. The appearance of agitated saline bubbles in the left heart chambers within three beats or less of their appearance in the right side of the heart is typically felt to represent the presence of an intracardiac shunt, most commonly a PFO. It is, however, important to remember that in patients with advanced heart failure and significantly elevated right-and left-sided atrial pressures there may not be a high enough gradient between both atria to cause a detectable shunt. Therefore, in addition to preoperative inspection for shunting, the intraoperative transesophageal echocardiogram should be used to confirm the presence or absence of any shunting, including a PFO that may not have been detected by transthoracic echocardiography. A PFO identified in patients undergoing CF-LVAD implantation should be closed at the time of surgery to eliminate the risk of hypoxemia from right to left shunting.
Follow-up Echocardiographic Assessment After Continuous-Flow Left Ventricular Assist Device Placement
After surgical implantation of a CF-LVAD, echocardiography continues to be an essential clinical tool for the ongoing evaluation and follow-up of pump function, native cardiac function, and overall patient care. The key elements of an echocardiogram in the patient supported with a CF-LVAD should focus on the following:
Evaluating Adequate Left Ventricular Unloading in Patients Supported by a Continuous-Flow Left Ventricular Assist Device
There are multiple features of the echocardiogram that can provide evidence of adequate LV unloading, which is invariably the primary hemodynamic goal of CF-LVAD support. These include the degree and frequency of aortic valve opening, the change in LV dimensions over time, and the cannula velocities.
Aortic Valve Opening
Evaluation of aortic valve opening by echocardiography is a simple, reliable way of determining adequate LV unloading in patients following CF-LVAD implantation [26]. In patients with CF-LVAD support, the aortic valve typically should open not every beat, but rather intermittently every two to three beats, or not at all. The frequency of aortic valve opening can be assessed by 2D imaging in the parasternal long-axis and short-axis views, as well as the apical three-chamber view. However, postsurgically image quality is frequently limited, and M-mode imaging through the aortic valve plane can assess the degree of leaflet opening and excursion more definitively (Figure 5). An aortic valve that opens consistently with each systole suggests that the left ventricle is not adequately unloaded and LV pressures remain significantly elevated. Common causes of inadequate LV unloading include uncontrolled hypertension, intravascular hypervolemia, any form of mechanical obstruction in the pump, including thrombus or kinking of the outflow graft, and the speed setting being too low. Table 3 summarizes the different clinical features of the most common causes of poor LV unloading.
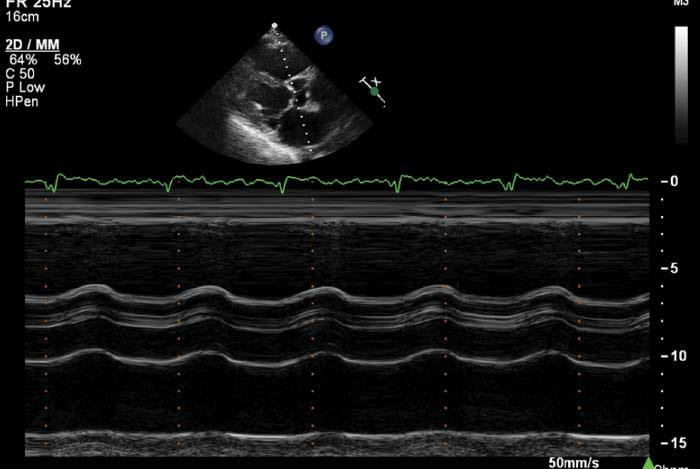
M-mode Image of the Aortic Valve from Patient with Continuous-flow Left Ventricular Assist Device Support.
Note the minimal aortic leaflet excursion indicative of adequate unloading of the left ventricle.
Causes of Poor Left Ventricular Unloading While the Patient has Continuous-flow Left Ventricular Assist Device Support.
| Condition | Clinical features | Management |
|---|---|---|
| Uncontrolled hypertension | Elevated PI/power, high RTF, may have persistent/worsening symptoms of left-sided heart failure | Increase vasodilator therapy |
| Hypervolemia | Elevated PI/power, JVD, peripheral edema, may have persistent/worsening symptoms of left-sided heart failure | Intensify diuresis |
| Mechanical obstruction | Persistent/worsening symptoms of left-sided heart failure, evidence of hemolysis is common (urine discoloration, increased LDH concentration) if secondary to thrombosis | CT angiography to evaluate the patient for the site of obstruction; if thrombus is suspected, intensify anticoagulation; urine alkalinization; pump exchange may be indicated |
| Pump speed too low | Persistent/worsening symptoms of left-sided heart failure | Increase pump speed, ideally under echocardiographic guidance |
CT, computed tomography; JVD, jugular venous distention; LDH, lactate dehydrogenase; PI, pulsatility index; RTF, return to flow.
Septal Morphology and Ventricular Dimensions
Follow-up echocardiographic assessment of LV chamber dimensions, together with the morphology and shape of the interventricular septum, can also provide information regarding the adequacy of LV unloading. Following initiation of CF-LVAD support, LV dimensions typically decrease and slight leftward motion of the interventricular septum is expected, reflecting a moderate decrease in LV pressures relative to RV pressures [27]. If, however, there is an interval increase of the LV dimension, or if there is a predominantly rightward motion of the septum, this suggests increased LV pressure relative to RV pressure, and is typically a sign of inadequate LV unloading. The differential is similar to that of an aortic valve that opens constantly, and workup for all of the previously mentioned scenarios should be pursued (Table 3).
Conversely, if the leftward septal shift is pronounced or associated with a dramatic reduction in LV cavity size, this highly suggests excessive LV decompression by the pump. Common causes of this include the pump speed being too high, significant recovery of LV systolic function, or any scenario of reduced LV preload, including intravascular hypovolemia or right-sided heart failure. Table 4 summarizes a diagnostic approach to help distinguish between these different scenarios, as well as potential therapeutic options.
Causes of Excessive Left Ventricular Decompression.
| Condition | Clinical features | Management |
|---|---|---|
| Pump speed too high | Frequent PI/suction events, low flow alarms, ventricular arrhythmias | Reduce pump speed, ideally under echocardiographic guidance |
| Hypovolemia | Frequent PI/suction events, ventricular arrhythmias, dry skin turgor, flat JVP, low RTF, preserved right ventricular function | Stop/reduce use of diuretics, encourage fluid intake, IV fluids if severe |
| Right-sided heart failure | JVD, peripheral edema, ascites with frequent PI/suction events, low flow alarms, ventricular arrhythmias, significant RV dilation/hypokinesis, worsening tricuspid regurgitation | Intensify use of diuretics, consider inotropes, speed reduction can be considered, if listed for transplant and meets criteria for status 1A |
| Recovery of LV function | Frequent PI/suction events, PI and flow values may vary, improved LVEF by echocardiography | Consider weaning patient off CF-LVAD support (over weeks to months). If tolerated can consider explantation |
CF-LVAD, continuous-flow left ventricular assist device; IV, intravenous; JVD, jugular venous distention; JVP, jugular venous pressure; LV, left ventricular; LVEF, left ventricular ejection fraction; PI, pulsatility index; RTF, return to flow; RV, right ventricular.
A ramp study should be considered whenever there is clinical evidence of poor LV unloading due to suspected mechanical obstruction [28]. This consists in measuring echocardiographic LV dimensions and the frequency of aortic valve opening while serially increasing the CF-LVAD pump speed. An inability to decrease LV dimensions or reduce the frequency of aortic valve opening despite increasing pump speeds should raise concern for mechanical obstruction in the pump, including pump thrombosis.
Cannula Velocities
The echocardiogram in patients supported with CF-LVADs should include attempts to measure blood flow velocities through the inflow cannula and outflow graft. However, Doppler measurements of inflow and outflow velocities are rarely interpretable in centrifugal-flow CF-LVADs because of multiple artifacts [29]. In axial-flow LVADs, inflow velocities are best evaluated in the apical windows, where flow is most parallel to the ultrasound beam. Outflow graft velocities can be measured in the parasternal long-axis window or the suprasternal window as blood flows out of the pump into the ascending aorta. There is no consensus on what the normal range of cannula or graft velocities should be. Ideally, there should be laminar flow by color Doppler imaging, with minimal turbulence, which suggests adequate alignment with mitral inflow. By spectral Doppler imaging, velocities vary widely depending on the patient and loading conditions, typically ranging between 0.3 and 1.5 m/s [30]. Most patients have some degree of phasic variation in velocity over the cardiac cycle, which reflects changes in flow through the pump due to native ventricular contraction and diastolic mitral inflow (Figure 6). Although there is not an established normal range of inflow cannula or outflow graft velocities, any significant change in these velocities over time warrants further evaluation and should be interpreted in the clinical context of the individual patient.
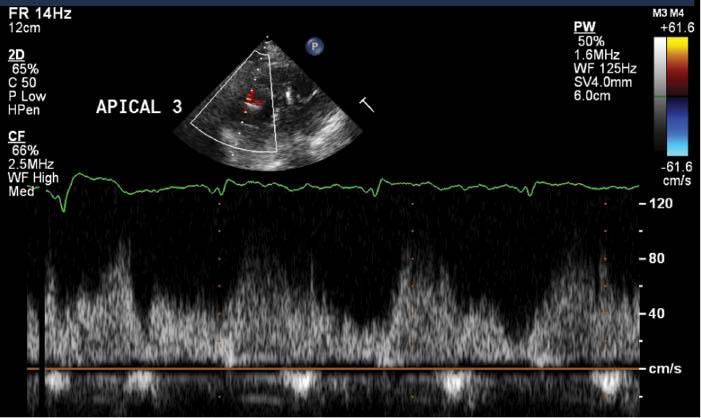
Inflow Cannula Velocity Measured from an Off-axis Apical Three-chamber View from a Patient with a Normally Functioning Continuous-flow Left Ventricular Assist Device.
Note the relatively low velocities, 30–80 cm/s, with phasic variation throughout the cardiac cycle reflecting some degree of native pulsatile flow.
Assessment of Right Ventricular Function
As discussed previously, it is crucial for patients supported by CF-LVADs to have relatively preserved RV function in order to maintain adequate right-sided cardiac output. Syndromes of right-sided heart failure can occur acutely in the early postoperative period or can have a more chronic presentation several years after implantation [31, 32]. Any interval worsening of RV dilation or the previously described indices of RV function support the diagnosis of RV failure after CF-LVAD implantation in the appropriate clinical context. In addition, echocardiography also provides a simple noninvasive way of detecting hemodynamics suggestive of RV decompensation.
Patients with right-sided heart failure after ventricular assist device (VAD) implantation typically experience a syndrome of RV volume overload as the right side of the heart struggles to match the effective forward flow provided by a CF-LVAD. As this progresses, the right ventricle becomes progressively dilated, functional tricuspid regurgitation worsens, and right-sided filling pressures become severely elevated. The echocardiographic estimation of right atrial and RV systolic pressure using the inferior vena cava diameter and tricuspid regurgitant jet velocity are well validated [8]. These methods appear to retain their accuracy in patients following CF-LVAD implantation [33]. Significant dilation of the inferior vena cava with minimal or no inspiratory collapse together with a relatively low tricuspid regurgitant jet velocity (less than 2.5 m/s in the setting of a dilated inferior vena cava) is the echocardiographic correlate of RV failure diagnosed by invasive hemodynamics, and highly suggests post–VAD implantation right-sided heart failure. The onset of RV failure after VAD implantation portends a poor prognosis and increased mortality. Treatment options are limited unless patients are candidates for heart transplantation or biventricular support [31]. Symptomatically, patients benefit from intravenous diuretics and initiation of inotropes for RV support. If septal morphology and ventricular chamber dimensions suggest excessive LV emptying and severe asymmetric dilation of the right ventricle relative to the left ventricle, a decrease in pump speed can also be helpful. Figure 7 illustrates images from a patient with post–VAD implantation right-sided heart failure.
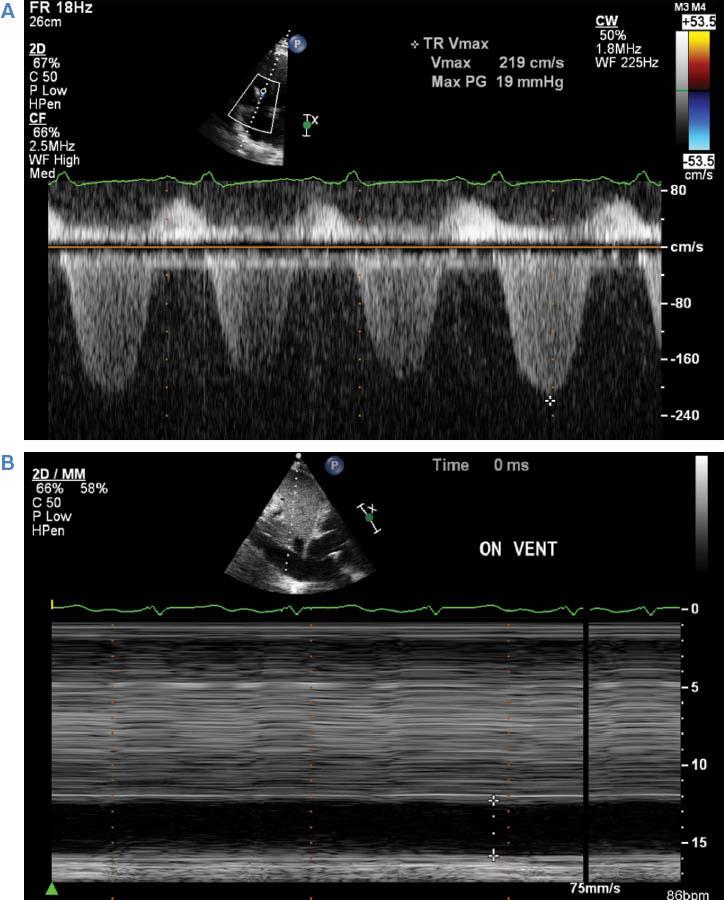
Tricuspid Regurgitation Doppler and Inferior Vena Cava M-mode Imaging from a Patient with Severe Right Ventricular Failure Following Continuous-flow Left Ventricular Assist Device Placement.
Note the combination of the low velocity of tricuspid regurgitation (A) and a severely dilated inferior vena cava with absence of inspiratory collapse (B), consistent with high right atrial pressure and low right ventricular contractile function.
Conclusion and Take-Home Message
The incidence of advanced heart failure patients who are potential candidates for mechanical support with CF-LVADs is steadily increasing worldwide. Echocardiography is a simple, noninvasive, yet highly useful diagnostic imaging modality that provides easily interpretable information that is instrumental in the care of patients supported by this relatively complex technology. It is crucial to remember that the utility of echocardiography is best when the images are understood in the clinical context of each individual patient, and decisions should never be made solely on the basis of the results of a single study.


